
5 курс / Пульмонология и фтизиатрия / Clinical_Tuberculosis_Friedman_Lloyd_N_,_Dedicoat
.pdf
340 The Surgical Management of Tuberculosis and Its Complications
(a)
(c)
(b) |
|
(d) |
Figure 17.25 (a) The chest x-ray of a patient with an intermittent sinus following thoracoplasty many years earlier, a space is seen (arrow) at the left base beneath the thoracoplasty. (b) After drainage, the extent of the cavity is seen, better demonstrated on CT scan. (d) Revision of the thoracoplasty dealt with the problem with no additional deformity.
and any abnormalities (e.g., bronchial stenosis) that may be present. Normally, for rigid bronchoscopy, patients are ventilated during the procedure using venturi jet insufflation. This is not done in MDR-TB cases; instead patients are kept apnoeic and the procedure is kept short with early return to facemask with waste gas scavenging. Bronchoscopy can be repeated if needed or alternatively the patients can be intubated using a single-lumen endotracheal tube, and the fiber-optic bronchoscopy can be passed via a catheter mount. Once again waste gases are removed using the anesthetic scavenging circuit.
After completion of the bronchoscopic procedures, the patient is intubated with a double-lumen endotracheal tube or, in challenging cases, with a single-lumen tube with bronchial blocker to allow single-lung ventilation. The operative side is now collapsed. Traditionally, in pulmonary surgery, many surgeons would often partially inflate the lung to facilitate dissection of the fissure or lung. However, in MDR-TB, the lung is kept collapsed ideally throughout the procedure until the final stages when the lung is re-inflated under water to check for air leaks prior to closure. This is done immediately prior to closing the
chest. The dissection of fissures or lung itself is performed on the deflated lung to avoid aerosolization and to minimize potential contamination of the surgical team. If, during the procedure, the operative side lung does need to be re-inflated to maintain adequate gas exchange, then this is co-ordinated with the surgeon and the incision is temporally covered while the lung is reinflated and then deflated.
As mentioned earlier, surgical dissection for TB can be particularly challenging and significant blood loss can occur. Our preference to avoid this is by very meticulous dissection predominantly using diathermy to divide adhesions and tissue rather than sharp dissection. We have also found that hemostatic agents— for example, chitosan (Celox™) and gelatin−thrombin matrix (Floseal™)—are useful adjuncts for control of bleeding. Attention to detail is essential combined with a thorough understanding of the anatomy and particularly constantly maintaining awareness of the anatomical relations at all times. This is especially important when the inter-lobar fissures have been obliterated by the disease process, and successful surgery may be dependent on being able to create a “new fissure” by dividing the lung parenchyma
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

References 341
and individually ligating vessels and bronchi until the pulmonary artery at the base of the fissure is identified. Special attention is paid to identifying and ligating the small bronchi to reduce any air leak and to prevent prolonged post-operative drainage.
REFERENCES
1.Peppas G, Molnar TF, Jeyasingham K, and Kirk AB. Thoracoplasty in the context of current surgical practice. Ann Thorac Surg. 1993;56:903–9.
2.Goldstraw P. Mediastinal exploration by mediastinoscopy and mediastinotomy. Br J Dis Chest. 1988;82:111–20.
3.Mouroux J, Venissac N, and Alifano M. Combined video-assisted mediastinoscopy and video-assisted thoracoscopy in the management of lung cancer. Ann Thorac Surg. 2001;72:1698–704.
4.Cybulsky IJ, and Bennett WF. Mediastinoscopy as a routine outpatient procedure. Ann Thorac Surg. 1994;58:176–8.
5.Navani N et al. EBUS-TBNA prevents mediastinocopies in the diagnosis of isolated mediastinal lymphadenopathy: A prospective trial.
Am J Respir Crit Care Med. 2012;186:255–60.
6.Mouroux J et al. Surgical management of pleuropulmonary tuberculosis. J Thorac Cardiovasc Surg. 1996;111:662–70.
7.Conlan AA, Lukanich JM, Shutz J, and Hurwitz SS. Elective pneumonectomy for benign lung disease: Modern day mortality and morbidity. J Thorac Cardiovasc Surg. 1995;110:1118–24.
8.Rizzi A et al. Results of surgical management of tuberculosis: Experience in 206 patients undergoing operation. Ann Thorac Surg. 1995;59:896–900.
9.Reed CE. Pneumonectomy for chronic infection: Fraught with danger? Ann Thorac Surg. 1995;59:408–11.
10.Goldstraw P. Surgery for pulmonary tuberculosis. Surgery. 1987;145:1071–82.
11.Griffiths EM, Kaplan DK, Goldstraw P, and Burman JF. Review of blood transfusion practices in thoracic surgery. Ann Thorac Surg. 1994;57:736–9.
12.Treasure RL, and Seaworth BJ. Current role of surgery in
Mycobacterium tuberculosis. Ann Thorac Surg. 1995;59:1405–7.
13.Langston HT. Thoracoplasty: The how and the why. Ann Thorac Surg. 1991;52:1351–3.
14.al-Kattan KM, Breach NM, Kaplan DK, and Goldstraw P. Softtissue reconstruction in thoracic surgery. Ann Thorac Surg. 1995;60:1372–5.
15.al-Kattan KM, and Goldstraw P. Completion pneumonectomy: Indications and outcome. J Thorac Cardiovasc Surg. 1995;110: 1125–9.
16.Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg. 2001;13(1):3–7.
17.Medical Research Council Cardiothoracic Epidemiology Group. National survey of notifications of tuberculosis in England and Wales in 1988. Thorax. 1992;47:770–5.
18.Pomerantz M, Madsen L, Goble M, and Iseman M. Surgical management of resistant Mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991;52:1108–12.
19.Marrone MT, Venkataramanan V, Goodman M, Hill AC, Jereb JA, and Mase SR. Surgical interventions for drug-resistant tuberculosis: A systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:6–16.
20.Yu JA, Weyant MJ, and Mitchell JD. Surgical treatment of atypical mycobacterial infections. Thorac Surg Clin. 2012;22(3):277–85.
21.Goble M et al. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–32.
22.Lawrence DR, Ohri SK, Moxon RE, and Fountain SW. Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg. 1997;64:1448–50.
23.Ferguson MK. Thoracoscopy for empyema, bronchopleural fistula, and chylothorax. Ann Thorac Surg. 1993;56:644–5.
24.Eloesser L. An operation for tuberculous empyema. Surg Gynaecol Obstet. 1935;60:1096.
25.Roberts CM, Citron KM, and Strickland BS. Intrathoracic aspergilloma: Role of CT in diagnosis and treatment. Radiology. 1987;165:123–8.
26.Massard G et al. Pleuropulmonary aspergilloma: Clinical spectrum and results of surgical treatment. Ann Thorac Surg. 1992;54:1159–64.
27.Daly RC et al. Pulmonary aspergillomas. Results of surgical treatment. J Thorac Cardiovasc Surg. 1986;92:981–8.
28.Remy J et al. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–7.
29.Jewkes J, Kay PH, Paneth M, and Citron KM. Pulmonary aspergilloma: Analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983;38:572–8.
30. Knott-Craig CJ et al. Management and prognosis of massive hemoptysis: Recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993;105:394–7.
31.Henderson AH, and Pearson JEG. Treatment of bronchopulmonary aspergillosis with observations on the use of natamycin. Thorax. 1968;23:519–23.
32.Rergkliang C et al. Surgical management of pulmonary cavity associated with fungus ball. Asian Cardiovasc Thorac Ann. 2004; 12(3):246–9.
33.Massard G, Olland A, Santelmo N, and Falcoz PE. Surgery for the sequelae of postprimary tuberculosis. Thorac Surg Clin. 2012;22:287–300.
34.Watanabe Y et al. Results in 104 patients undergoing bronchoplastic procedures for bronchial lesions. Ann Thorac Surg. 1990;50:607–14.
35.Frist WH, Mathisen DJ, Hilgenberg AD, and Grillo HC. Bronchial sleeve resection with and without pulmonary resection. J Thorac Cardiovasc Surg. 1987;93:350–7.
36.Chow TT, Kwan A, Lin Z, and Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64:371–8.

Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

18
Tuberculosis in Childhood and Pregnancy
LINDSAY H. CAMERON AND JEFFREY R. STARKE
Tuberculosis in children
Tuberculosis in pregnant women and the newborn References
TUBERCULOSIS IN CHILDREN
Introduction
Childhood tuberculosis differs from adult tuberculosis in epidemiology, clinical and radiographic presentation, and treatment. The risk of a child developing tuberculosis is influenced by age, immune status, and the intensity of exposure to a source case with tuberculosis disease.1,2
Much of adult pulmonary tuberculosis is caused by a reactivation of Mycobacterium tuberculosis bacilli contained in the apices of the lungs following hematogenous dissemination at the time of infection. Childhood tuberculosis is most often a complication of the pathophysiologic events following the initial infection. The interval between infection and disease is often years in immunocompetent adults but is often only weeks to months in young children.1 Young children rarely develop contagious pulmonary disease and are more likely than adults to develop extrapulmonary manifestations of tuberculosis. Based on the differences in the pathophysiology of tuberculosis and the clinical manifestations between adults and children, the approaches to the diagnosis, treatment, and prevention of tuberculosis infection and disease in children are different.3
TERMINOLOGY
The terminology used to describe the various stages and clinical presentations of childhood tuberculosis follow the pathophysiology from the initial transmission of M. tuberculosis through infection and disease. The stages may not be completely distinct in young children.
Exposure occurs when a child has had significant contact with an adult or adolescent source case with potentially contagious pulmonary tuberculosis. The likelihood of an exposure leading to infection in a child is associated with the contagiousness of the source case and the child’s proximity and duration of exposure. The most frequent location for exposure of a child is her household, but it can occur at school, a day care center, or other closed
343
361
366
setting. In this stage, the initial test of infection (either a tuberculin skin test [TST] or interferon gamma release assay [IGRA]) is negative, the child lacks signs and symptoms of disease and the chest radiograph is normal. Some children may have inhaled droplet nuclei containing M. tuberculosis and have early infection, which is unapparent to a clinician as it takes 2–3 months for a TST or IGRA to become positive. A contact investigation—evaluat- ing those close to the suspected source case with tuberculosis—is the most important activity in a community to prevent cases of tuberculosis in children.4,5 The US Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics (AAP) recommend that children younger than 4 years of age and HIV (human immunodeficiency virus)-infected children at any age who are exposed to a source case with tuberculosis be evaluated for tuberculosis infection and be started on treatment to prevent rapid development of disseminated or meningeal tuberculosis, which can occur before the TST or IGRA become reactive.6,7
Infection occurs when a child inhales droplet nuclei containing M. tuberculosis, which are deposited in the lung and are established intracellularly within the lung parenchyma and/or the draining lymph system. The hallmark of this stage is a reactive TST or IGRA in an asymptomatic child. The child’s chest radiograph is either normal or reveals only granuloma or calcification in the lung parenchyma and/or regional lymph nodes. In developed countries, all children infected with M. tuberculosis are treated to prevent the development of disease in the near or distant future.
Disease occurs when a child develops signs and symptoms or radiographic manifestations caused by M. tuberculosis. Not all infected individuals will develop disease. An immunocompetent adult with untreated tuberculosis infection has a 5%–10% chance of tuberculosis infection progressing to disease; one-half of this risk occurs within 2–3 years of infection. In contrast, up to 50% of immunocompetent infants with untreated tuberculosis infection will develop disease which is often serious and life threatening, most often 6-9 months after the initial exposure.1 Children typically have closed caseous lesions with relatively few mycobacteria (termed paucibacillary disease) and, until adolescence, lack the tussive force to transmit bacteria in their environment.
343

344 Tuberculosis in Childhood and Pregnancy
This contributes to the complexity in confirming a diagnosis of childhood tuberculosis; compared to adults, most children have both acid-fast sputum smear and culture negative tuberculosis. Therefore, most children are diagnosed with tuberculosis disease based on a known exposure to tuberculosis, a positive test of infection, clinical symptoms, and supportive radiographic studies. In contrast, adolescents may develop adult-type or reactivation disease with a high burden of bacilli that can be easily transmitted into the environment.
Infection and the onset of tuberculosis disease are often distinct events in adolescents and adults, separated in time. In young children, tuberculosis disease often complicates the initial infection, suggesting that the two stages occur on a continuum.8 This can cause confusion regarding the optimal treatment regimen for young children with tuberculosis. The current consensus in the United States is to consider disease to be present if thoracic adenopathy or other concerning chest radiographic findings caused by M. tuberculosis are identified, even if the child is asymptomatic.
Epidemiology
As most children with tuberculosis infection and disease acquire M. tuberculosis from an adult in their environment, the epidemiology of childhood tuberculosis tends to follow that in adults. The risk of a child acquiring tuberculosis infection is environmental, determined by the likelihood that she will be in contact with an adult with contagious disease. In contrast, the risk of a child infected with M. tuberculosis progressing to disease depends mainly on host immunologic and genetic factors.
CHILDHOOD TUBERCULOSIS WORLDWIDE
Tuberculosis has surpassed HIV/AIDS as the most frequent infectious disease cause of death worldwide.9 Approximately 95% of tuberculosis cases occur in the developing world. The global burden of tuberculosis is influenced by several factors including: the HIV pandemic, the development of multidrug-resistant tuberculosis (MDR-TB), and the disproportionately low access of populations in low-resource settings worldwide to both diagnostic tests and effective medical therapy. Due to a lack of laboratory infrastructure in low-resource settings, few children have culture-con- firmed tuberculosis, which leads to variation in country-specific childhood tuberculosis case reporting.
The first estimate of the global burden of childhood tuberculosis was published by the WHO in 2012. More recently, mathematical models have been used to estimate the global burden of childhood tuberculosis infection and disease.9–11 At least 1 million children develop tuberculosis disease each year, and 233,000 die from complications of tuberculosis.9,10,12 The youngest and most vulnerable children are at highest risk of death from tuberculosis. According to more recent reports from the WHO, nearly 650 children die each day, 80% of whom are less than 5 years of age.13 The highest overall numbers of childhood tuberculosis cases occur in Southeast Asia followed by Africa. The proportion of pediatric cases is higher in Sub-Saharan Africa than in any other area of the world.14 India has the largest number of newly diagnosed childhood tuberculosis cases worldwide, followed by the People’s
Republic of China. Vietnam has had the greatest recent increase in reported rates.
The incidence of drug-resistant tuberculosis has increased in some areas of the world in both adults and children. MDR-TB is defined as resistance to at least isoniazid and rifampin; extensively drug-resistant tuberculosis (XDR-TB) includes MDR-TB plus resistance to any fluoroquinolone and at least one of the three injectable drugs (kanamycin, capreomycin, or amikacin). In 2014, the worldwide estimate for MDR-TB in children was 2.9% of all cases, and for XDR-TB it was 0.1%. The highest incidence of MDR-TB in children occur in the Southeast Asian, African, and Western Pacific Regions; however, the proportion of drugresistant cases is highest in countries belonging to the Russian Federation, where over 30% of children with tuberculosis have a drug-resistant organism.11
CHILDHOOD TUBERCULOSIS IN THE UNITED STATES
Tuberculosis case rates in the United States decreased during the first half of the twentieth century, long before the advent of antituberculosis drugs, as a result of improved living conditions and, likely, genetic selection favoring persons resistant to developing disease. A resurgence of tuberculosis in the late 1980s was associated primarily with: the HIV epidemic; transmission of the organism in congregate settings including health-care institutions; disease occurring in recent immigrants; and poor conduct of community tuberculosis management, specifically contact tracing. Since 1992, the tuberculosis incidence in the United States has decreased, however, the case rate has remained stable at approximately 3 cases per 100,000 persons.15 From 2007 to 2017, 121,209 tuberculosis cases had complete data reported to the National Tuberculosis Surveillance System, of whom 9,276 (8%) were children and adolescents less than 18 years of age.16 The incidence rate of tuberculosis among children and adolescents was 1 case per 100,000 person-years and declined by 47.8% from 2007 to 2017. The tuberculosis incidence rates were highest in children less than 12 months of age (1.9 per 100,000 person-years), followed by adolescents aged 15–17 years (1.4 per 100,000 years), and were lowest among children aged 7–12 years (0.5 per 100,000 person-years).
From 2010 to 2017, non-US born children, children born to non-US-born parents, and children of racial or ethnic minority status were disproportionately affected by tuberculosis.16 The age-specific incidence rate of non-US-born children was 13 times that of US-born children. Non-US-born children accounted for approximately 32% of the total number of childhood tuberculosis cases, the majority being from Mexico followed by Ethiopia, the Philippines, Myanmar, and Haiti. In US-born children, the incidence rates of tuberculosis if both parents were non-US-born and if one parent was non-US-born were 8 and 3 times as high, respectively, compared to US-born children with both parents being US-born. This is supported by prior research that found among the US-born children with tuberculosis that 75% had some international connection through a family member or previous travel or residence in a tuberculosis endemic country.17,18 Similar to adults in the United States, tuberculosis occurs disproportionately among children of racial-ethnic minority status. The incidence rates of tuberculosis among children of Native Hawaiian or Pacific Islander, Asian, Native American or Native Alaskan, black,
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Tuberculosis in children 345
and Hispanic children were 144, 44, 22, 19, and 18 times as high, respectively, as among non-Hispanic white children.16
The rates of drug-resistant tuberculosis in children in the United States remain low.18,19 A total of 89 childhood cases of MDR tuberculosis were reported in the United States in 2015; of those, 70.8% were non-US-born. Among children with cultureconfirmed tuberculosis in the United States in 2015, 15.2% had organisms with resistance to at least one first-line drug and 0.9% had MDR organisms.
CHILDHOOD TUBERCULOSIS INFECTION
Modeling studies estimate that 75,000,000 children under the age of 15 years worldwide are infected with M. tuberculosis.10,11,13 In high-prevalence countries, rates of tuberculosis infection among the young population average 20%–50%. However, reliable estimates in these countries are difficult to obtain due to lack of diagnostic resources, which has led to an underdiagnosis and underreporting of infected children.
In the United States, only a few states consider tuberculosis infection to be a reportable condition. Most children are infected in the home; however, outbreaks of childhood and adolescent tuberculosis infection and disease continue to occur in schools, day care centers, and other congregate settings. The most efficient method of identifying children with recent tuberculosis infection is through contact tracing of adults with contagious pulmonary tuberculosis.3,5,6 On average, 30%–50% of household contacts of an index case will have a positive test of infection.
TUBERCULOSIS IN HIV-INFECTED CHILDREN
Most cases of tuberculosis in HIV-infected children occur in developing countries. A study conducted in South Africa demonstrated that the incidence of tuberculosis disease in HIVinfected children is 42 times higher than that in HIV-uninfected children.20 Establishing the diagnosis of tuberculosis in an HIVinfected child may be difficult because the immune based tests (TST or IGRA) may be negative and many other HIV-related opportunistic infections and conditions have overlapping clinical features with tuberculosis.21,22 HIV-infected children suffer from more severe and progressive forms of tuberculosis, and more often have disease in extrapulmonary sites, including meningitis and abdominal disease.23–26 Radiographic findings are similar to those in children with normal immune systems, but lobar disease and lung cavitation are more common. The most common symptoms include fatigue, malaise, weight loss, nonspecific respiratory symptoms, and fever. Tuberculosis is often suspected when a HIV-infected child has symptom persistence after treatment for a community-acquired pneumonia. Recurrent tuberculosis disease and relapse occur more frequently in HIV-infected children, and they are also at increased risk to have primary or secondary drug resistance.
The mortality rate of HIV-infected children with untreated M. tuberculosis is high and correlates with their CD4 counts. The host immune response to tuberculosis infection appears to enhance HIV replication and accelerate the immune suppression caused by HIV.27,28 Increased mortality rates are often attributable to progressive HIV infection rather than tuberculosis. Therefore, HIVinfected children with potential tuberculosis exposures or recent
documented infection should be promptly evaluated and treatment for tuberculosis infection should be started. Conversely, all children with suspected tuberculosis should be tested for HIV.10 In general, HIV-infected children with tuberculosis have a favorable prognosis as long as they do not have severe or progressive tuberculosis and appropriate antituberculosis drugs and antiretroviral therapy are available.18
Childhood tuberculosis transmission
The diagnosis of a case of childhood tuberculosis is a sentinel event in a community. Children are most often infected with M. tuberculosis by an adult or adolescent in their immediate household with contagious tuberculosis disease, most often a parent, grandparent, older sibling, or aunt/uncle. Transmission from an extrafamilial source case occurs but is much less common and has been reported by babysitters, school teachers, music teachers, school bus drivers, church attendees, classmates, gardeners, and candy store keepers leading to hundreds of mini epidemics within population groups.29 A study from Texas Children’s Hospital in Houston, Texas reported that when chest radiographs were obtained of adult care takers of children admitted with suspected tuberculosis, 15% had previously undetected pulmonary tuberculosis.30 In the household of an adult with smear positive, cavitary tuberculosis, infants and toddlers are frequently infected. Adolescents are also at increased risk of becoming infected, while school-aged children are less often affected.1,31 Once an adult has been started on antituberculosis therapy, their risk of transmitting M. tuberculosis to children in their environment is low. Adults with smear and culture positive tuberculosis are assumed to be infectious for at least 2 weeks after the start of effective chemotherapy. Those with chronic tuberculosis who go undetected, or those who are inadequately treated, pose the highest risk of transmitting M. tuberculosis, including the transmission of drugresistant bacteria.
Early observational studies of young children in orphanages with pulmonary tuberculosis revealed that children rarely, if ever, infect other children.32 Young children often lack the tussive force to expectorate bacilli into their environment. Children who were more likely to transmit M. tuberculosis were found to have imaging findings most consistent with adult-type tuberculosis.33 Siblings, parents, or nurses who care for children with pulmonary tuberculosis often remain TST or IGRA negative.34 When M. tuberculosis has been documented in children’s hospitals, it is invariably due to transmission from an adult caregiver of a child with undiagnosed pulmonary tuberculosis.35–37 Guidelines issued by the CDC state that most children with typical tuberculosis do not require isolation in the hospital unless they have uncontrolled productive cough, a cavitary lesion, or acid-fast smear positive sputa.38 Tuberculosis in childhood must be considered in the transmission of tuberculosis in a community, not due to the likelihood of their transmitting infectious droplets into their immediate environment, but rather because, if untreated, they can harbor a partially healed infection that lies dormant, which remains at risk of reactivating as contagious pulmonary tuberculosis many years later. This most often occurs during times of emotional or physiologic stress including: adolescence, pregnancy, immunosuppression, or

346 Tuberculosis in Childhood and Pregnancy
old age. Children with tuberculosis infection constitute a longlasting reservoir of tuberculosis in a community. Programs targeting children for prevention and treatment of tuberculosis will have a limited impact on immediate disease rates in a community, but a profound impact on the long-term control of the disease.3
Childhood tuberculosis pathogenesis and immunology
In over 95% of cases of childhood tuberculosis, the portal of entry for M. tuberculosis is the lung.39 Respiratory particles larger than 10 µm containing tubercle bacilli are often caught by the mucociliary clearance mechanisms of the bronchial tree and are expectorated with cough. Small particles are easily inhaled beyond the bronchial tree into the alveoli of the lung parenchyma. The primary complex of tuberculosis consists of local disease at the portal of entry and the regional lymph nodes. While primary infection most often occurs in the lung, it can occur in any location in the body.40 Ingestion of infected milk with bovine tuberculosis can lead to a gastrointestinal primary lesion. Infection of the skin or mucous membrane can occur through a skin abrasion, laceration, or an insect bite. The number of bacilli necessary to establish an infection in children is unknown, but is thought to be only a few organisms.
The time period from entrance of the tubercle bacilli to a person’s development of cutaneous hypersensitivity is usually 2–12 weeks, most often 4–8 weeks. The onset of hypersensitivity in children may be accompanied by upper respiratory congestion, cough, and fever that can last 1–3 weeks. During this phase, there is an intensified tissue reaction, and the primary complex may be evident on chest radiograph. The primary focus increases in size during this time, but is not encapsulated. As hypersensitivity evolves, the inflammatory response becomes more intense and the regional lymph nodes often enlarge. The parenchymal portion of the primary complex undergoes caseous necrosis and encapsulation, and can either continue to enlarge or heal completely by fibrosis or calcification. If the parenchymal lesion continues to enlarge, it may result in focal pneumonitis and thickening of the underlying pleura or intense caseation may occur in the center of the lesion with liquefaction and extension into the adjacent bronchus, leaving a residual primary tuberculosis cavity.39,40
During the development of the primary parenchymal lesion and accelerated caseation brought on by the development of hypersensitivity, tubercle bacilli from the primary complex spread via the bloodstream and lymphatics throughout the body. The areas most commonly seeded are the apices of the lungs, liver, spleen, meninges, peritoneum, lymph nodes, pleura, and bone. If large numbers of bacilli are disseminated, a child is at risk of developing miliary (disseminated) tuberculosis. When small numbers of bacilli are disseminated, microscopic foci are scattered to various tissues. These areas are the most common extrapulmonary sites for childhood or adult onset tuberculosis disease (months to years later).40
The tubercle foci in the regional lymph nodes develop some fibrosis and encapsulation, but the healing in this location is often less complete than in parenchymal lesions. Viable M. tuberculosis bacilli may persist for decades after calcification of these nodes.
The size of these lymph nodes remains normal in most cases of primary tuberculosis infection. If these lymph nodes enlarge during the host inflammatory reaction, due to their location, hilar and paratracheal lymph nodes may exert external pressure on a regional bronchus. Partial obstruction caused by external compression leads to hyperinflation of the distal lung segment. Complete obstruction may result in atelectasis of the entire lung segment.2,41,42 These children often present with respiratory distress or failure and can have audible wheeze or diminished breath sounds on pulmonary examination. More often, caseous lymph nodes attach to and erode through the bronchial wall and can transmit infection to the lung parenchyma leading to bronchial obstruction and atelectasis. The resultant radiographic finding is referred to as “epituberculosis,” “collapse-consolidation,” and “segmental” tuberculosis. Rarely, intrathoracic tuberculous nodes invade adjacent structures, such as the pericardium or esophagus.
The timeline for primary tuberculosis and its complications in infants and children is well documented.40 Widespread lymphohematogenous dissemination leading to miliary (disseminated) tuberculosis, including tuberculous meningitis, occurs in 0.5%– 2% of infected children usually 2–6 months after initial infection. Intrathoracic manifestations generally occur within the first year. Clinically apparent lymph node or endobronchial tuberculosis usually appears within the first 3–9 months. Musculoskeletal lesions often take 12 months to develop; renal lesions may not present clinically for 5–25 years after initial infection.
Tuberculosis disease that occurs more than a year after the primary infection is thought to be secondary to endogenous regrowth of persistent bacilli from the primary infection and subclinical dissemination. Exogenous reinfection rarely results in tuberculosis disease; most cases of postprimary or reactivation tuberculosis in adolescents are believed to be secondary to endogenous organisms. Reactivation tuberculosis affects female adolescents twice as often as males; the reason for their increased risk is unknown but likely related to physiologic and hormonal changes during this time period. The most common disease manifestation of tuberculosis disease is a pulmonary infiltrate or cavitary lesion in the lung apices, where oxygen tension is high. Dissemination of bacilli during reactivation is uncommon in immunocompetent adolescents.
The age of the child at the time of acquisition of tuberculosis infection is the most influential factor on their development of both primary and reactivation tuberculosis. Disseminated (miliary and tuberculous meningitis), lymph node and subsequent segmental disease complicating primary infection occur most often in young children. Approximately 50% of untreated children less than 12 months of age develop radiographically significant tuberculosis disease, compared with 24% of children 1–10 years of age and 16% of children aged 11–15 years.43,44 If young children do not suffer from early complications following primary infection, their risk of developing reactivation tuberculosis later in life is low. Conversely, older children and adolescents rarely experience complications following primary infection but have a greater risk of developing reactivation pulmonary tuberculosis in adolescence or adulthood.
There is increasing knowledge on the primary immune response to tuberculosis. As shown in children with underlying
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Tuberculosis in children 347
immunodeficiency, immune control of mycobacteria is dependent oncell-mediatedimmunity(M.tuberculosisspecificT-lymphocytes, macrophages, monocytes, neutrophils, dendritic cells, Toll-like receptors, INF-γ, TNF [tumor necrosis factor]-α, and interleu- kin-2).45,46 The increased risk of progression from infection to disease that exists among young children and adolescents compared to school-age children is thought to be due to an insufficient production and function of Toll-like receptors, dendritic cells, macrophages in addition to a deficient ability for CD4+ cells to express Th1 effector function. This immune dysfunction is highest among neonates, young infants, and immunosuppressed children. As the immune system matures or becomes functional (with immune reconstitution), the risk of progression to disease decreases.
Clinical manifestations and diagnosis of childhood tuberculosis
The timing of the diagnosis of tuberculosis in children differs between highand low-burden settings.47 In high-burden countries, most children are passively diagnosed when they present for medical care with symptomatic tuberculosis disease.48 Tuberculosis is suspected in a symptomatic child when a household or adult contact also has tuberculosis. In these settings, most children are diagnosed based on clinical symptoms, without radiologic data or microbiologic confirmation. To aid in the diagnosis of tuberculosis in children in resource limited settings, a variety of scoring systems have been devised based on clinical signs and symptoms, known epidemiologic exposure, and the available tests.49 However, the sensitivity and specificity of these systems varies by location, leading to both overand underdiagnosis of childhood tuberculosis. No clinical scoring system has been adequately validated in a clinical trial.
Since 2013, the WHO has published guidelines for intensive case-finding strategies (active case-finding) in high-burden countries, which improves the identification of both adults and children with tuberculosis.50 Several countries have implemented this practice, which includes screening close contacts of those with symptomatic tuberculosis, those who are HIV infected, and those with poor access to health-care services. There are published screening algorithms for children, although the most common practice is an interview to identify those at risk (including symptoms screen and HIV testing).50 Active case-finding strategies improve rates of diagnosis of tuberculosis in a community, allows for earlier diagnosis and is cost-effective.51–53
In low-burden countries, children with tuberculosis are discovered one of three ways: they present for medical care with symptomatic tuberculosis, they are diagnosed during contact tracing, or are diagnosed through a communityor school-based testing program. The most cost-effective method to identify children at risk for tuberculosis is through contact tracing for an adult case. In the United States, up to 50% of children with pulmonary tuberculosis are diagnosed during contact tracing. The affected child typically has few or no symptoms, but the investigation reveals a positive test of infection (TST or IGRA) and an abnormal chest radiograph.
CHILDHOOD TUBERCULOSIS DISEASE: CLINICAL MANIFESTATIONS
Pulmonary
The primary pulmonary complex includes the parenchymal pulmonary focus and the regional lymph nodes.38 Approximately 70% of lung foci are subpleural, and localized pleurisy is common. The initial parenchymal inflammation is not usually visible on chest radiograph, but a localized, nonspecific infiltrate may be seen before the development of tissue hypersensitivity. During the initial infection, all lobar segments of the lung are at equal risk of being involved, and 25% of cases have multiple primary lung foci.54 The hallmark of primary tuberculosis in the lung is the relatively large size of the regional lymphadenitis compared with the relatively small size of the initial lung focus. As delayedtype hypersensitivity develops, the hilar lymph nodes continue to enlarge in some children, especially infants, compressing the regional bronchus and causing obstruction. The usual sequence is hilar lymphadenopathy, focal hyperinflation, and then atelectasis. The resulting radiographic shadows have been called collapseconsolidation or segmental tuberculosis (Figure 18.1). Inflamed caseous nodes may attach to the endobronchial wall and erode through it, causing endobronchial tuberculosis or a fistulous tract.55 The caseum causes complete obstruction of the bronchus, resulting in extensive infiltrate and collapse.56,57 The radiographic picture is similar to that caused by the aspiration of a foreign body but is usually quite different from bacterial or viral pneumonia. In pulmonary tuberculosis, the lymph nodes act as the “foreign body.” Enlargement of the subcarinal lymph nodes can cause compression of the esophagus and, rarely, a bronchoesophageal fistula.58
Most cases of tuberculous bronchial obstruction in children resolve fully with appropriate treatment.59 There is occasionally a residual calcification of the primary focus or regional lymph nodes. The appearance of calcification implies that the lesion has been present for at least 6–12 months. Healing of the segment can be complicated by scarring or contraction associated with cylindrical bronchiectasis, but this is rare.
Children can have lobar pneumonia without impressive hilar lymphadenopathy. If the primary infection is progressively
Figure 18.1 Collapse-consolidation/segmental tuberculosis.
348 Tuberculosis in Childhood and Pregnancy
Figure 18.2 Miliary tuberculosis.
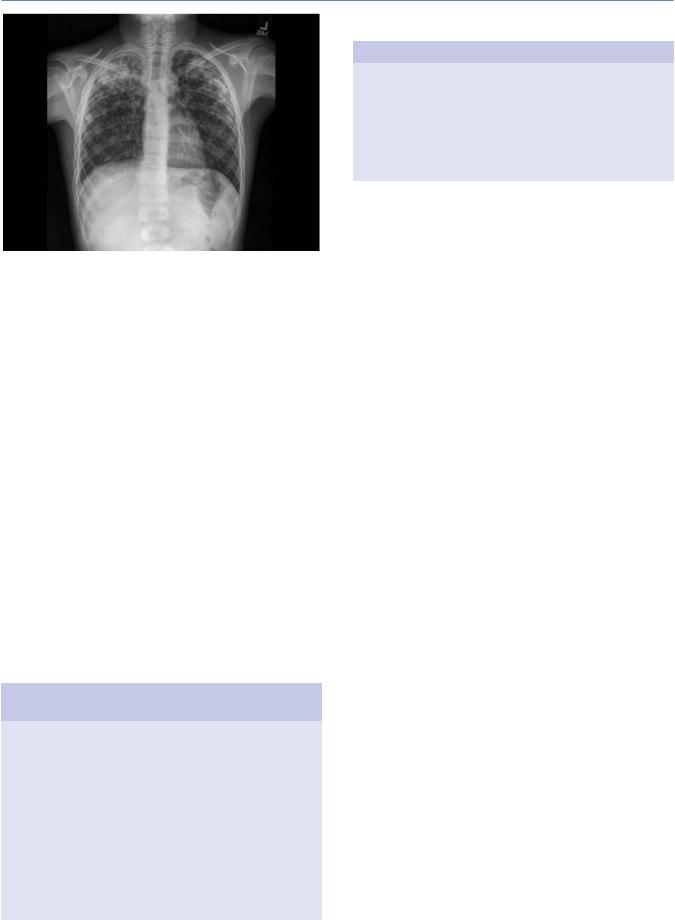
destructive, liquefaction of the lung parenchyma can lead to the formation of a thin-walled primary tuberculosis cavity. Bullous tuberculous lesions rarely occur in the lungs and, if they rupture, can lead to pneumothorax. Erosion of a parenchymal focus of tuberculosis into a blood or lymphatic vessel can result in dissemination of the bacilli and a miliary pattern, with small nodules evenly distributed on the chest radiograph (Figure 18.2).
The symptoms and physical signs of primary pulmonary tuberculosis in children are surprisingly meager considering the degree of radiographic changes often present. The physical manifestations of disease and associated symptoms differ by age of onset (Table 18.1). Young infants and adolescents are more likely to experience signs and symptoms of tuberculosis, while school-age children are more likely to have clinically silent disease. When active case finding is performed, up to 50% of infants and children with radiographically moderate to severe pulmonary tuberculosis have no physical findings. Nonproductive cough and mild dyspnea are the most common symptoms in infants, which is probably due to their smaller airway diameters relative to the parenchymal and lymph node changes in pulmonary tuberculosis. Systemic symptoms such as fever, night sweats, anorexia, and decreased activity are less
Table 18.1 Symptoms and signs of pulmonary tuberculosis in childhood
|
Infants and young |
Older children |
Symptom or sign |
children |
and adolescents |
Fever |
Common |
Uncommon |
Night sweats |
Rare |
Uncommon |
Cough |
Common |
Common |
Productive cough |
Rare |
Common |
Hemoptysis |
Never |
Rare |
Dyspnea |
Common |
Rare |
Rales |
Common |
Uncommon |
Wheezing |
Common |
Uncommon |
Dullness to |
Rare |
Uncommon |
percussion |
|
|
Diminished breath |
Common |
Uncommon |
sounds |
|
|
Table 18.2 Comparison of chest radiographs of pulmonary tuberculosis in adults and children
Characteristic(s) |
Adults |
Children |
Location |
Apical |
Anywhere (25% |
|
|
multilobar) |
Adenopathy |
Rare |
Usual |
Cavitation |
Common |
Rare (except in |
|
|
adolescence) |
Signs and |
Consistent |
Relative paucity |
symptoms |
|
|
common. Some infants present with poor weight gain or develop failure to thrive, which may not improve until several months into their treatment course. Pulmonary signs are even less common. Some infants and young children with bronchial obstruction have signs from air trapping such as localized wheezing or decreased breath sounds that may be accompanied by tachypnea or, rarely, respiratory distress. The pulmonary symptoms and signs occasionally are alleviated by antibiotics, suggesting a bacterial superinfection distal to the focus of tuberculous bronchial obstruction, which contributes to the clinical presentation of disease.
As expected, the radiographic findings in childhood tuberculosis reflect the pathophysiology and are different from the findings in adults (Table 18.2).60 The majority of cases of pulmonary tuberculosis in children resolve radiographically with or without antituberculosis chemotherapy; however, in the pretreatment era, up to 60% of children had residual anatomic sequelae not apparent on radiographs. With delayed or no treatment, calcification of the caseous lesions is common. Healing of the pulmonary segment can be complicated by scarring or contraction associated with cylindrical bronchiectasis or bronchostenosis. When these complications occur in the upper lobes they are usually clinically silent. They are rare in children who have successfully completed current regimens of chemotherapy.61
A rare but serious complication of tuberculosis in a child occurs when the primary focus enlarges steadily and develops a large caseous center.62 Liquefaction can cause formation of a primary cavity associated with a high burden of tubercle bacilli. The radiographic and clinical picture of progressive primary tuberculosis is closest to that of bronchial pneumonia. The child commonly presents with high fever, moderate to severe cough with sputum production, weight loss, and night sweats. Physical signs include diminished breath sounds, rales, and dullness to percussion over the cavity. The enlarging focus can slough necrotic debris into the adjacent bronchus, leading to further intrapulmonary dissemination. Rupture of this cavity into the pleural space causes a bronchopleural fistula or pyopneumothorax. Rupture into the pericardium can cause acute pericarditis with constriction. Prior to the advent of antituberculosis chemotherapy, the mortality of progressive pulmonary tuberculosis in children was 30%–50%; with current treatment, the prognosis is excellent for full recovery.
Pulmonary tuberculosis in adults usually represents endogenous reactivation of a site of tuberculosis infection previously established in the body. This form of tuberculosis is rare in childhood but can occur in adolescence.40 Children with a healed
Книга в списке рекомендаций к покупке и прочтению сайта https://meduniver.com/

Tuberculosis in children 349
tuberculosis infection acquired when they were younger than 2 years of age rarely develop reactivation pulmonary disease, which is more common in those who acquire the initial infection when they are older than 7 years of age.62 The most common pulmonary sites are the original parenchymal focus, lymph nodes, or the apical seedings (Simon foci) established during the hematogenous phase of the early infection.63 This form of tuberculosis disease usually remains localized in the lungs, as the established immune response prevents further extrapulmonary spread. The most common radiographic findings are extensive infiltrates or thick-walled cavities in the upper lobes.
Older children and adolescents with reactivation tuberculosis are more likely to experience fever, anorexia, malaise, weight loss, night sweats, productive cough, hemoptysis, and chest pain compared to children with primary pulmonary tuberculosis. However, physical examination findings often are minor or absent, even when cavities or large infiltrates are present. Most symptoms improve within several weeks of starting effective treatment, although cough may last for several months. This form of tuberculosis usually remains localized to the lungs, however, the child may be highly contagious if there is significant sputum production and cough. The prognosis for full recovery is excellent with appropriate therapy.
Pleural
Tuberculous pleural effusions are caused by the hypersensitivity response to the discharge of bacilli into the pleural space from a subpleural pulmonary focus or from subpleural caseous lymph node.62 The discharge is usually small and the subsequent pleuritis is localized and asymptomatic. Larger and clinically significant effusions occur months to years after the primary infection. Often the radiographic abnormality is more extensive than would be suggested by physical findings or symptoms (Figure 18.3). A clinically significant pleural effusion occurs in 10%–30% of tuberculosis cases in young adults, is infrequent in children younger than 6 years of age, and rare in children younger than 2 years of age.63 Tuberculous pleural effusions are usually unilateral in children. They are rarely associated with a segmental pulmonary lesion and are uncommon in disseminated tuberculosis.
Figure 18.3 Pleural effusion.
The onset of symptoms and signs usually is abrupt with fever, chest pain, shortness of breath, dullness to percussion, and diminished breath sounds on the affected side. The fever can be high and may last for several weeks after the initiation of antituberculosis chemotherapy. The diagnosis may be difficult to make as the acid-fast stain of the pleural fluid is nearly always negative and the culture is positive in only 30%–50% of cases. The best material for the diagnosis is a pleural biopsy, which will reveal caseating granulomas on pathologic examination in up to 90% of cases, and up to 70% are culture positive. The prognosis is excellent, but radiographic resolution often takes months.
Lymphohematogenous (disseminated) disease
Tubercle bacilli are disseminated to distant anatomic sites in virtually all cases of asymptomatic tuberculosis infection.39,44,62 Autopsy evaluation of persons who died of other causes within days to weeks after an initial tuberculosis infection demonstrated organisms in many organs and tissues, most commonly the liver, spleen, skin, and lung apices. The clinical manifestations produced by lymphohematogenous tuberculosis depends on the quantity of organisms released from the primary focus and the host immune response. Children with an impaired immune response, including infants or those who are HIV infected, are more likely to develop severe forms of disseminated disease.
The occult dissemination of tubercle bacilli during the initial infection usually produces no symptoms, but it is the event that results in extrapulmonary foci that can become the site of disease month to years after the initial infection. Children rarely experience protracted hematogenous tuberculosis caused by the intermittent release of tubercle bacilli as a caseous focus erodes through the wall of a blood vessel in the lung. The clinical picture may be acute, but more often it is indolent and prolonged, with spiking fever accompanying the release of organisms into the bloodstream. Multiple organ involvement is common, leading to hepatomegaly, splenomegaly, lymphadenitis in superficial or deep nodes, and papulonecrotic tuberculids appearing on the skin. Bones and joints or kidneys also can be involved. Meningitis occurs only late in the course and was the main cause of death in the prechemotherapy era. Pulmonary involvement is surprisingly mild early in the course, but diffuse involvement becomes apparent if treatment is not initiated early. Culture confirmation of this complication can be difficult; bone marrow or liver aspirate stains and cultures may be necessary and should be performed if the diagnosis is considered and other diagnostic testing is unrevealing.
The most common clinically significant form of disseminated tuberculosis is miliary disease, which occurs when massive numbers of tubercle bacilli are released into the bloodstream, leading to disease in two or more organs.44,63,64 Miliary tuberculosis is most often a complication of primary infection, occurring within 2–6 months of the primary inoculation. While this form of disease is most common in those who are immunosuppressed or in infants and young children, it also occurs in adolescents and older adults, resulting from the breakdown of a previously healed or calcified primary pulmonary lesion.
The clinical manifestations of miliary tuberculosis are protean and depend on the load and final location of disseminated
