
KAPLAN_USMLE_STEP_1_LECTURE_NOTES_2018_BIOCHEMISTRY_and_GENETICS
.pdf
Part I ● Biochemistry
Stage |
Carbohydrate |
|
|
|
Protein |
|
Fat |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
I |
Glucose |
|
|
|
Amino acids |
|
Fatty acids |
|||
|
|
|
|
|
|
|
|
|
|
|
II |
|
|
|
Pyruvate |
|
|
|
|
|
|
|
|
|
|
Acetyl-CoA |
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
III |
|
|
|
TCA |
|
||||
|
|
|
|
Cycle |
2 CO2 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
3 NADH & FADH2 |
O2 |
||||||
IV |
|
e– |
|
ETC |
|
|
|
|
|
|
|
|
|
|
|
H2O |
|||
|
|
|
ATP synthase |
||||||
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
ADP + Pi ATP |
|
||||||
|
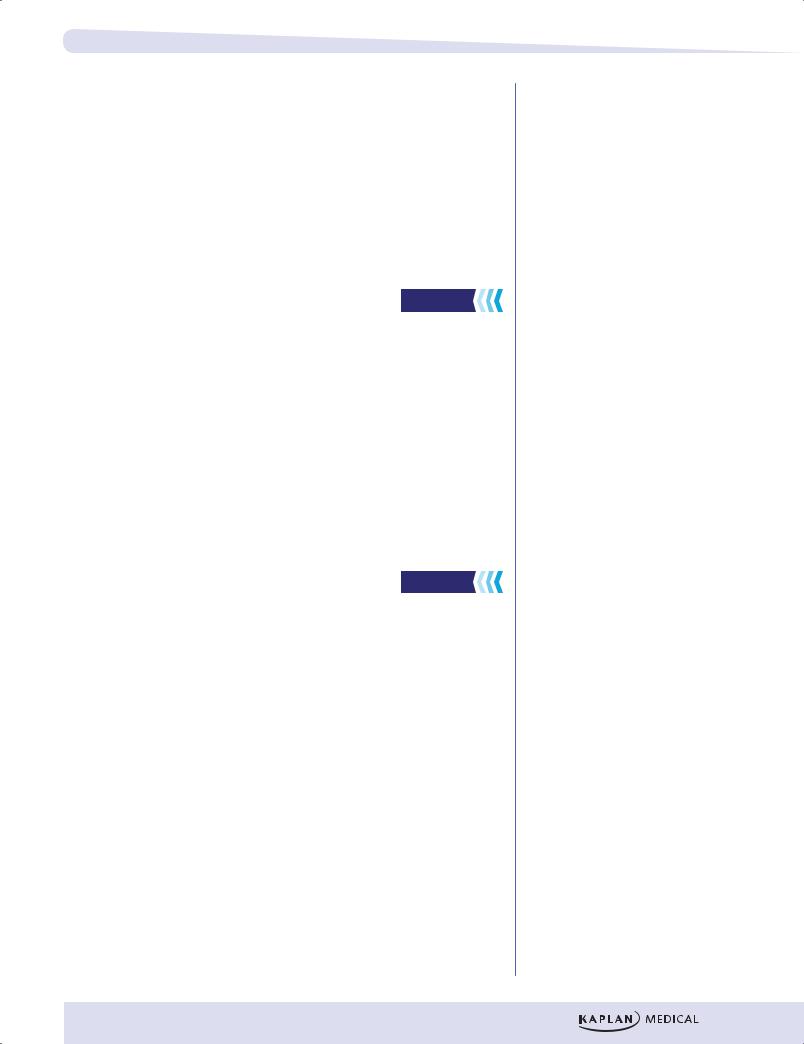
Figure I-11-1. Energy from Metabolic Fuels |
|
|||||||
METABOLIC ENERGY STORAGE
ATP is a form of circulating energy currency in cells. It is formed in catabolic pathways by phosphorylation of ADP and may provide energy for biosynthesis (anabolic pathways). There is a limited amount of ATP in circulation. Most of the excess energy from the diet is stored as fatty acids (a reduced polymer of acetyl CoA) and glycogen (a polymer of glucose). Although proteins can be mobilized for energy in a prolonged fast, they are normally more important for other functions (contractile elements in muscle, enzymes, intracellular matrix, etc.).
In addition to energy reserves, many other types of biochemicals are required to maintain an organism. Cholesterol is required for cell membrane structure, proteins for muscle contraction, and polysaccharides for the intracellular matrix, to name just a few examples. These substances may be produced from transformed dietary components.
REGULATION OF FUEL METABOLISM
The pathways that are operational in fuel metabolism depend on the nutritional status of the organism. Shifts between storage and mobilization of a particular fuel, as well as shifts among the types of fuel being used, are very pronounced in going from the well-fed state to an overnight fast, and finally to a prolonged state of starvation. The shifting metabolic patterns are regulated mainly by the insulin/glucagon ratio. Insulin is an anabolic hormone which promotes fuel
164

Part I ● Biochemistry
|
|
|
|
|
RED |
|
Pyruvate |
Glucose |
Glucose |
|
|
|
|
|
CELL |
|
ATP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bile |
|
|
Lactate |
|
|
|
|
|
|
Bile salts |
Cholesterol |
Lactate |
LIVER |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fatty |
Acetyl |
|
Pyruvate |
Glucose |
Glucose |
|
|
|
|
acids |
CoA |
|
|||
|
|
|
|
|
|
|
|
||
|
|
|
Glycerol-P |
|
Urea |
|
|
||
|
|
|
|
Fat |
CO2 |
GLYCOGEN |
Glucose |
||
|
|
|
|
|
ATP |
Amino acids |
|
||
|
|
|
|
Glycerol VLDL |
|
Amino acids |
Blood |
Pyruvate |
|
FAT |
Fatty |
|
|
|
Acetyl |
||||
|
|
|
|
|
|
|
|||
|
acids |
|
|
|
|
|
|
|
|
|
|
|
|
Chylo- |
|
|
Amino |
|
CoA |
Glycerol-P |
Acetyl |
|
|
Acetyl CoA |
PROTEIN |
CO2 |
|||
|
|
microns |
acids |
||||||
|
CoA |
CO2 |
Pyruvate |
CO2 |
|
ATP |
|||
Pyruvate |
|
||||||||
ATP |
|
BRAIN |
|||||||
|
|
|
|||||||
|
|
ATP |
|
Glucose |
|
GLYCOGEN |
|||
|
Glucose |
|
Glucose |
|
|
||||
ADIPOSE TISSUE |
|
|
|
|
MUSCLE |
|
|
||
|
|
|
|
|
|
|
|||
|
|
|
|
|
Glucose |
|
|
|
|
|
|
|
Figure I-11-2. Metabolic Profile of the Well-Fed (Absorptive) State |
|
|||||
166

Chapter 11 ● Energy Metabolism
|
|
|
RED |
Pyruvate |
Glucose |
|
|
|
|
CELL |
|
ATP |
|
|
|
|
|
|
|
|
|
|
|
|
Lactate |
CORI CYCLE |
|
|
|
LIVER |
|
Lactate |
Glycerol-P |
|
|
|
|
|
|
||
|
|
Fatty |
Acetyl |
Pyruvate |
Glucose |
Glucose |
|
|
acids |
CoA |
|||
|
|
|
|
|
||
|
|
Glycerol-P |
CO2 |
Urea |
|
|
|
|
ATP |
|
Glucose |
||
|
|
|
Alanine |
GLYCOGEN |
||
|
|
|
Ketone |
|
||
|
Glycerol |
|
bodies |
|
|
Pyruvate |
|
|
|
|
|
||
|
Fatty |
Fatty acid |
Ketone |
Alanine |
Blood |
Acetyl |
FAT |
bodies |
|||||
acids |
albumins |
|
|
|
CoA |
|
|
|
|
|
Amino |
|
CO2 |
|
Acetyl |
|
Ketone |
PROTEIN |
ATP |
|
|
|
acids |
|
|||
|
CoA |
|
bodies |
|
|
|
|
CO2 |
|
Fatty |
|
|
BRAIN |
|
|
acids |
|
|
|
|
|
ATP |
|
Acetyl |
CO2 |
|
|
ADIPOSE TISSUE |
|
CoA |
ATP |
|
|
|
|
|
MUSCLE |
|
|||
|
|
|
|
|||
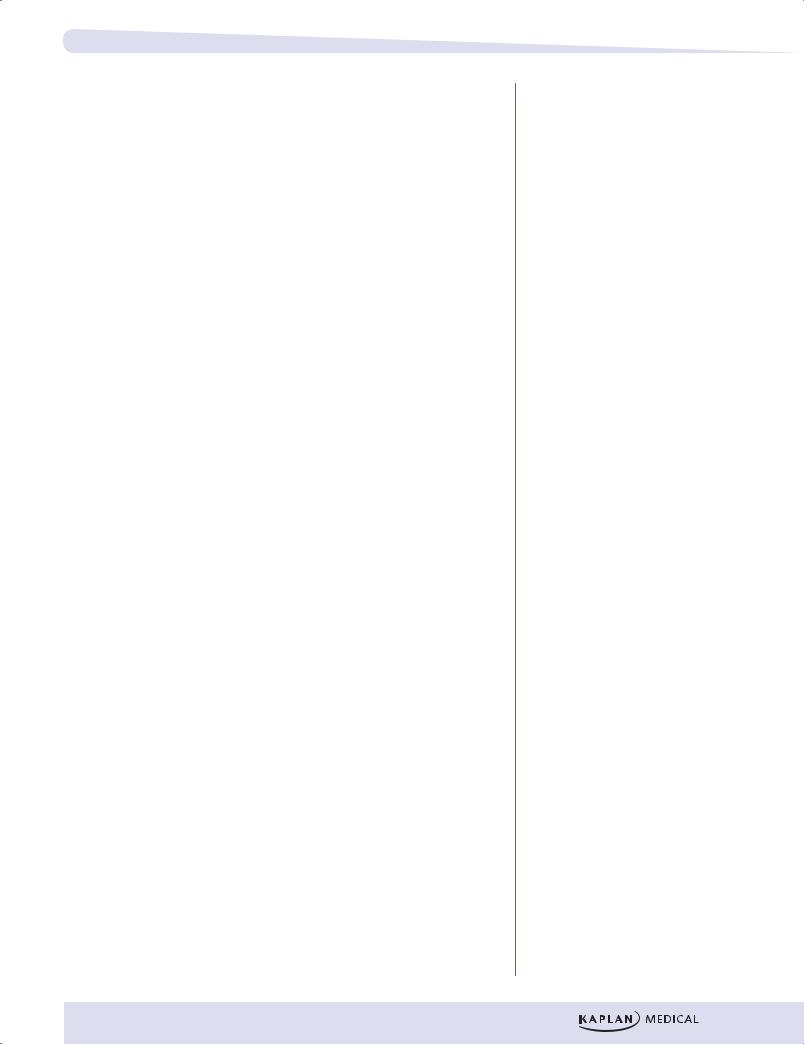
Figure I-11-3. Metabolic Profile of the Postabsorptive State
167


Chapter 11 ● Energy Metabolism
•The increase in insulin after a meal stimulates both glycogen synthesis and fatty acid synthesis in liver. The fatty acids are converted to triglycerides and released into the blood as very low-density lipoproteins (VLDLs). In the well-fed state, the liver derives most of its energy from the oxidation of excess amino acids.
•Between meals and during prolonged fasts, the liver releases glucose into the blood. The increase in glucagon during fasting promotes both glycogen degradation and gluconeogenesis.
•Lactate, glycerol, and amino acids provide carbon skeletons for glucose synthesis.
Adipose Tissue
After a meal, the elevated insulin stimulates glucose uptake by adipose tissue. Insulin also stimulates fatty acid release from VLDL and chylomicron triglyceride (triglyceride is also known as triacylglycerol).
•Lipoprotein lipase, an enzyme found in the capillary bed of adipose tissue, is induced by insulin.
•The fatty acids that are released from lipoproteins are taken up by adipose tissue and re-esterified to triglyceride for storage.
•The glycerol phosphate required for triglyceride synthesis comes from glucose metabolized in the adipocyte.
•Insulin is also very effective in suppressing the release of fatty acids from adipose tissue.
•During the fasting state, the decrease in insulin and the increase in epinephrine activate hormone-sensitive lipase in fat cells, allowing fatty acids to be released into the circulation.
Recall Question
In a prolonged state of starvation, which of the following is the major source of energy for muscles?
A.Fatty acids
B.Glucose
C.Glycogen
D.Ketones
Answer: A
169
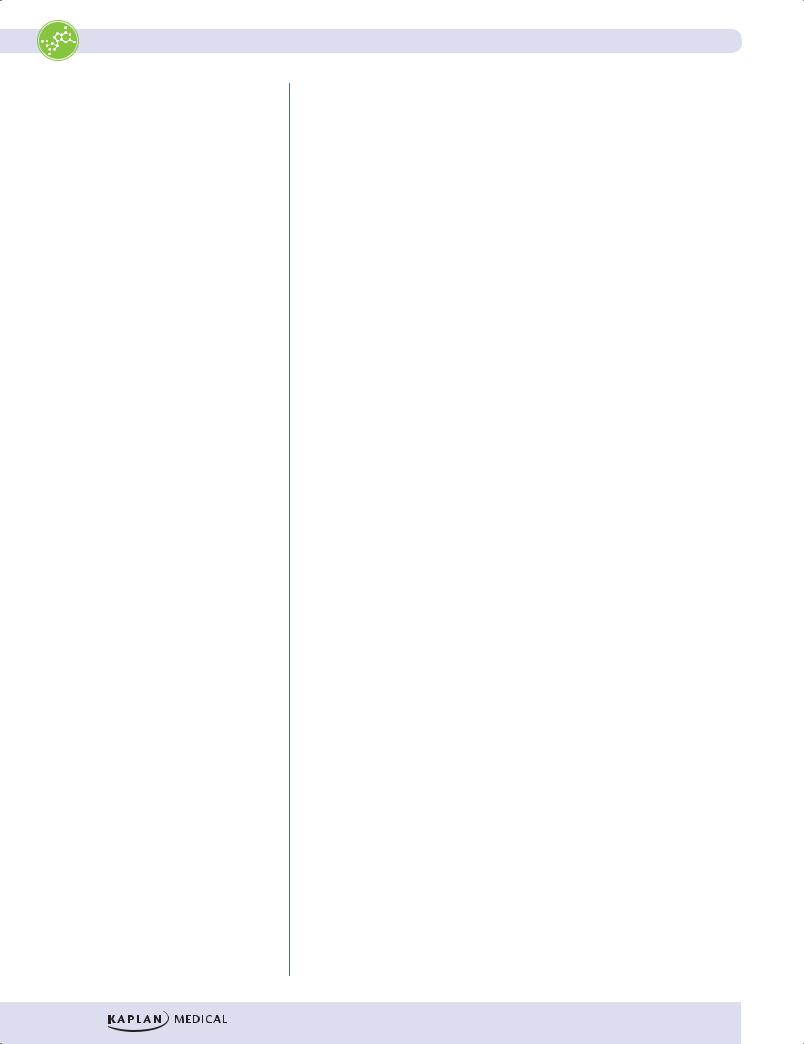

Chapter 11 ● Energy Metabolism
Brain
Although the brain represents 2% of total body weight, it obtains 15% of the cardiac output, uses 20% of total O2, and consumes 25% of the total glucose. Therefore, glucose is the primary fuel for the brain.
•Blood glucose levels are tightly regulated to maintain the concentration levels that enable sufficient glucose uptake into the brain via GLUT 1 and GLUT 3 transporters.
•Because glycogen levels in the brain are minor, normal function depends upon continuous glucose supply from the bloodstream.
•In hypoglycemic conditions (<70 mg/dL), centers in the hypothalamus sense a fall in blood glucose level, and the release of glucagon and epinephrine is triggered.
•Fatty acids cannot cross the blood–brain barrier and are therefore not used at all.
•Between meals, the brain relies on blood glucose supplied by either hepatic glycogenolysis or gluconeogenesis. Only in prolonged fasts does the brain gain the capacity to use ketones for energy, and even then ketones supply only approximately 2/3 of the fuel; the remainder is glucose.
171

Part I ● Biochemistry
Review Questions
Select the ONE best answer.
1.Two weeks after an episode of the flu, an 8-year-old boy with IDDM is brought to the emergency room in a coma. His breathing is rapid and deep, and his breath has a fruity odor. His blood glucose is 36.5 mM (normal: 4–6 mM [70–110 mg/dL]). The physician administers IV fluids, insulin, and potassium chloride. A rapid effect of insulin in this situation is to stimulate
A.gluconeogenesis in the liver
B.fatty acid release from adipose
C.glucose transport in muscle
D.ketone utilization in the brain
E.glycogenolysis in the liver
2.An alcoholic has been on a 2-week drinking binge during which time she has eaten little and has become severely hypoglycemic. Which additional condition may develop in response to chronic, severe hypoglycemia?
A.Glycogen accumulation in the liver with cirrhosis
B.Thiamine deficiency
C.Ketoacidosis
D.Folate deficiency
E.Hyperuricemia
3.After a routine physical exam and blood work, a woman with a normal weight for her height was advised that her lipid profile showed an elevation of blood triglycerides. The doctor advises the patient to lower fat consumption which disappoints her since she avidly consumes whole milk. The woman consults a nutritionist, who states that whole milk is 3.5% fat, which corresponds to approximately 11 g of fat in an 8 ounce serving. If she switches to drinking skim milk (nonfat), approximately how many additional grams of carbohydrates should she consume to make up for the loss of fat in the 8 ounce serving?
A.5 grams
B.11 grams
C.15 grams
D.25 grams
E.35 grams
172
Chapter 11 ● Energy Metabolism
Answers
1.Answer: C. Insulin increases glucose transport in only two tissues, adipose and muscle. The major site of glucose uptake is muscle, which decreases hyperglycemia. Glucose and ketone transport and metabolism are insulin independent in the brain (choice D). Insulin would slow gluconeogenesis (choice A) and fatty acid release from adipose (choice B). Insulin would inhibit glycogenolysis in the liver (choice E).
2.Answer: C. Severe hypoglycemia lowers the insulin level and increases glucagon. This would favor fatty acid release from the adipose and ketogenesis in the liver.
3. Answer: D. You are expected to know that carbohydrates have 4 Kcal/gram, proteins have 4 Kcal/gram, fat has 9 Kcal/gram, and alcohol has 7 Kcal/gram. In this question, 11 grams of fat times 9 Kcal/gram = 99 Kcal which is rounded to 100 Kcal. Dividing 100 Kcal by 4 Kcal/gram of carbohydrate is 25 grams.
173
