Part I ● Biochemistry
Double bonds in fatty acids are in the cis- configuration. Trans- double bonds are unnatural and predominate in fatty acids found in margarine and other foods where partial hydrogenation of vegetable oils is used in their preparation. Compared with liquid oils, these partial hydrogenated fatty acids are conveniently solid at cool temperatures. When incorporated into phospholipids that constitute membranes, trans-fatty acids decrease membrane fluidity, similar to saturated fatty acids that are found in butter fat and other foods. Trans-fatty acids, as well as saturated fatty acids, are associated with increased risk of atherosclerosis.
Activation of Fatty Acids
When fatty acids are used in metabolism, they are first activated by attaching coenzyme A (CoA); fatty acyl CoA synthetase catalyzes this activation step. The product is generically referred to as a fatty acyl CoA or sometimes just acyl CoA. Specific examples would be acetyl CoA with a 2-carbon acyl group, or palmitoyl CoA with a 16-carbon acyl group.
Fatty acid + CoA + ATP → Fatty acyl CoA + AMP + PPi
LIPID DIGESTION
Typical high-fat meals contain gram-level amounts of triglycerides and milli- gram-level amounts of cholesterol and cholesterol esters.
•Upon entry into the intestinal lumen, bile is secreted by the liver to emulsify the lipid contents.
•The pancreas secretes pancreatic lipase, colipase, and cholesterol esterase which degrade the lipids to 2-monoglyceride, fatty acids, and cholesterol. These lipids are absorbed and re-esterified to tryglycerides and cholesterol esters and packaged, along with apoprotein B-48 and other lipids (e.g., fat-soluble vitamins), into chylomicrons.
•Normally, there is very little lipid loss in stools. Defects in lipid digestion result in steatorrhea, in which there is an excessive amount of lipids in stool (fatty stools).
FATTY ACID BIOSYNTHESIS
Excess dietary glucose can be converted to fatty acids in the liver and subsequently sent to the adipose tissue for storage. Adipose tissue synthesizes smaller quantities of fatty acids. The pathway is shown in Figure I-15-1. Insulin promotes many steps in the conversion of glucose to acetyl CoA in the liver:
•Glucokinase (induced)
•PFK-2/PFK-1 (PFK-2 dephosphorylated)
•Pyruvate dehydrogenase (dephosphorylated)
Both of the major enzymes of fatty acid synthesis are also affected by insulin:
•Acetyl CoA carboxylase (dephosphorylated, activated)
•Fatty acid synthase (induced)
224

Chapter 15 ● Lipid Synthesis and Storage
Citrate Shuttle and Malic Enzyme
The citrate shuttle transports acetyl CoA groups from the mitochondria to the cytoplasm for fatty acid synthesis. Acetyl CoA combines with oxaloacetate in the mitochondria to form citrate, but rather than continuing in the citric acid cycle, citrate is transported into the cytoplasm. Factors that indirectly promote this process include insulin and high-energy status.
In the cytoplasm, citrate lyase splits citrate back into acetyl CoA and oxaloacetate. The oxaloacetate returns to the mitochondria to transport additional acetyl CoA. This process is shown below and includes the important malic enzyme. This reaction represents an additional source of cytoplasmic NADPH in liver and adipose tissue, supplementing that from the HMP shunt.
Mitochondria |
|
|
|
|
Cytoplasm |
|
|
|
|
|
|
|
|
|
Citrate |
|
|
|
|
|
Insulin |
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Induces |
|
|
|
|
|
|
|
shuttle |
|
|
|
|
|
|
|
|
|
CO2 |
|
|
|
|
|
|
|
|
Acetyl CoA |
|
|
|
|
|
|
|
Acetyl CoA |
|
|
Citrate |
|
|
Citrate |
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
carboxylase |
|
|
|
|
Fatty acid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acetyl CoA |
(biotin) |
|
|
|
|
|
synthase |
|
Fatty acid |
|
|
|
|
|
|
|
|
|
|
|
|
Malonyl |
|
|
|
|
palmitate |
|
|
|
|
|
|
|
|
|
|
|
|
NADPH |
|
|
OAA |
|
|
OAA |
|
|
|
|
CO2 |
|
CoA |
|
|
|
(16:0) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PDH |
|
Pyruvate |
|
|
Malate |
|
|
|
NADP+ |
|
|
|
|
|
|
|
|
|
|
|
carboxylase |
|
|
|
|
Malic |
|
|
|
|
|
|
|
|
|
|
|
|
|
(biotin) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
enzyme |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NADPH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HMP shunt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyruvate |
|
|
|
Pyruvate |
|
|
|
|
|
Glucose |
|
|
|
|
|
|
and Glycolysis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure I-15-1. Synthesis of Palmitate from Glucose
Acetyl CoA is activated in the cytoplasm for incorporation into fatty acids by acetyl CoA carboxylase, the rate-limiting enzyme of fatty acid biosynthesis. Acetyl CoA carboxylase requires biotin, ATP, and CO2. Controls include:
•Activation by insulin (dephosphorylated)
•Activation by citrate
The CO2 added to form malonyl CoA is never incorporated into the fatty acid because it is removed by fatty acid synthase during the addition of the acetyl group to the fatty acid.
Fatty Acid Synthase
Fatty acid synthase is more appropriately called palmitate synthase because palmitate is the only fatty acid that humans can synthesize de novo. This enzyme is a large, multienzyme complex in the cytoplasm that is rapidly induced in the
225
Chapter 15 ● Lipid Synthesis and Storage
|
ADIPOSE |
|
|
|
|
|
|
|
|
|
|
LIVER |
DHAP |
|
Glucose |
|
|
|
|
|
|
|
Glucose |
|
|
|
DHAP |
|
|
|
Glucose |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Glycerol 3-P |
|
|
|
|
|
|
|
Glycerol 3-P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
dehydrogenase |
|
|
dehydrogenase |
|
|
|
|
|
|
|
Glycerol kinase |
|
|
|
|
|
|
|
|
|
|
|
|
Glycerol |
|
|
|
|
Glycerol 3-P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Glycerol 3-P |
|
|
|
|
|
|
|
|
|
|
3 FA CoA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 FA CoA |
|
|
|
VLDL |
|
|
|
VLDL |
|
|
|
|
|
|
|
|
|
Triglyceride |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Triglyceride |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(storage) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure I-15-2. Glycerol 3-P Dehydrogenase and
Glycerol Kinase in Triglyceride Synthesis and Storage
Glycerophospholipids are used for membrane synthesis and for producing a hydrophilic surface layer on lipoproteins such as VLDL. In cell membranes, they also serve as a reservoir of second messengers such as diacylglycerol, inositol 1,4,5-triphosphate, and arachidonic acid.
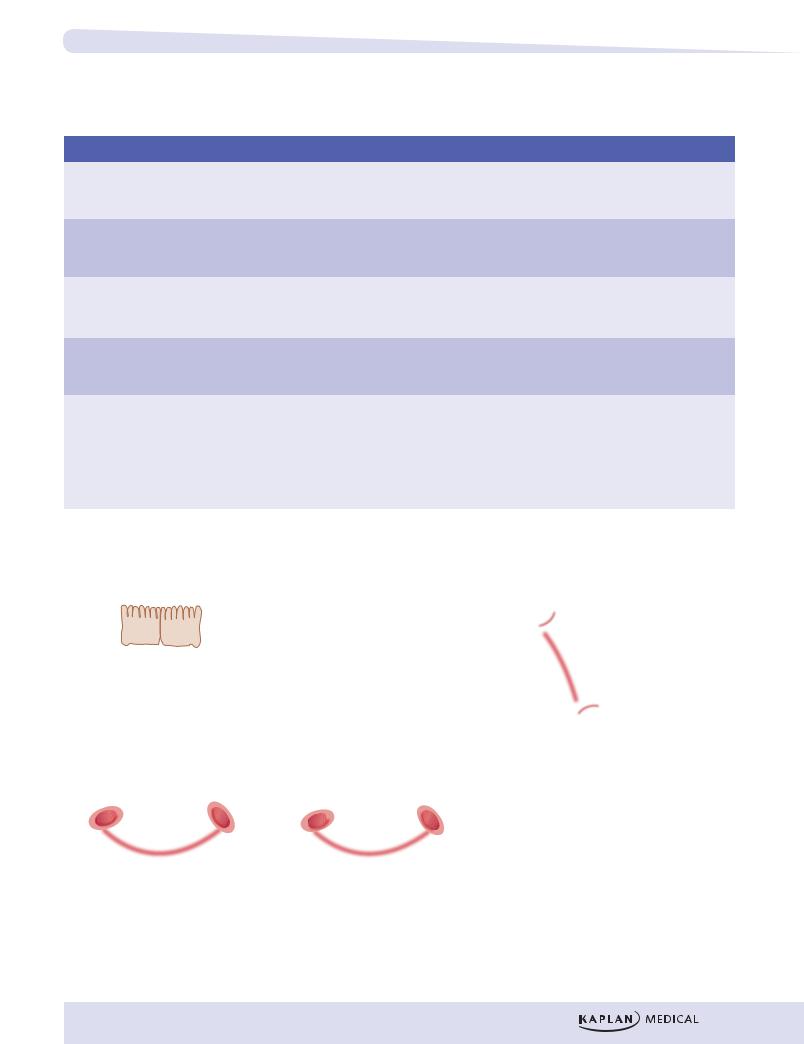
The structure of glycerophospholipids is similar to that of triglycerides, except that the last fatty acid is replaced by phosphate and a water-soluble group such as choline (phosphatidylcholine, lecithin) or inositol (phosphatidylinositol).
Triglyceride
AcidFatty AcidFatty AcidFatty
Glycerol
Glycerophospholipid
Glycerol 3P
3P
Inositol
Figure I-15-3. Triglycerides and
Glycerophospholipids
Part I ● Biochemistry
LIPOPROTEIN METABOLISM
Cholesterol Digestion
Triglycerides and cholesterol are transported in the blood as lipoproteins. Lipoproteins are named according to their density, which increases with the percentage of protein in the particle.
From least dense to most dense:
Chylomicrons < VLDL < IDL (intermediate-density lipoproteins) < LDL (low-density lipoproteins) < HDL (high-density lipoproteins)
VLDL secreted from the liver
Phospholipid


 Cholesterol
Cholesterol
Triacylglycerol
Apoprotein B-100 




Figure I-15-4. Lipoprotein Structure
228
Lipoprotein and Apoprotein Classes
Chapter 15 ● Lipid Synthesis and Storage
High-Yield
Table I-15-1. Classes of Lipoproteins and Important Apoproteins
|
Lipoprotein |
|
|
Functions |
|
|
Apoproteins |
|
|
Functions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chylomicrons |
|
Transport dietary triglyceride and |
|
apoB-48 |
|
Secreted by intestine |
|
|
|
|
cholesterol from intestine to tissues |
|
apoC-II |
|
Activates lipoprotein lipase |
|
|
|
|
|
|
|
apoE |
|
Uptake of remnants by the liver |
|
|
|
|
|
|
|
|
|
|
|
|
|
VLDL |
|
Transports triglyceride from |
|
apoB-100 |
|
Secreted by liver |
|
|
|
|
liver to tissues |
|
apoC-II |
|
Activates lipoprotein lipase |
|
|
|
|
|
|
|
apoE |
|
Uptake of remnants (IDL) by liver |
|
|
|
|
|
|
|
|
|
|
|
|
|
IDL |
|
Picks up cholesterol from HDL |
|
apoE |
|
Uptake by liver |
|
(VLDL remnants) |
|
to become LDL |
|
apoB-100 |
|
|
|
|
|
|
|
Picked up by liver |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LDL |
|
Delivers cholesterol into cells |
|
apoB-100 |
|
Uptake by liver and other tissues via |
|
|
|
|
|
|
|
|
|
|
LDL receptor (apoB-100 |
|
|
|
|
|
|
|
|
|
|
receptor) |
|
|
|
|
|
|
|
|
|
|
|
|
|
HDL |
|
Picks up cholesterol accumulating |
|
apoA-1 |
|
Activates lecithin cholesterol |
|
|
|
|
in blood vessels |
|
|
|
|
acyltransferase (LCAT) to produce |
|
|
|
|
Delivers cholesterol to liver and |
|
|
|
|
cholesterol esters |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
steroidogenic tissues via scavenger |
|
|
|
|
|
|
|
|
|
|
receptor (SR-B1) |
|
|
|
|
|
|
|
|
|
|
Shuttles apoC-II and apoE in blood |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
~ 80% |
HDL |
Released from |
Dietary fat |
|
|
~ 20% |
liver and small |
|
|
|
LDL |
intestine (A, C, E) |
|
Liver |
|
|
Intestine |
|
(B-100) |
|
Dietary Endogenous |
Hepatic |
|
|
LCAT |
|
cholesterol |
lipase |
|
|
|
|
|
Extra hepatic tissues
Chylomicrons |
Remnants |
VLDL |
IDL |
(E, C-II, B-48) |
|
(E, B-48) (E, C-II, B-100) |
(E, B-100) |
LP Lipase (Fatty |
|
acid) |
LP Lipase (Fatty |
|
acid) |
|
|
|
|
|
|
|
|
Adipose tissue and muscle |
Adipose tissue and muscle |
HDL (cholesterol ester-rich)
CETP
Deliver cholesterol
to liver and steroidogenic tissues via SR-B1
LCAT = lecithin cholesterol acyltransferase CETP = cholesterol ester transfer protein SR-B1 = scavenger receptor B1
Figure I-15-5. Lipoprotein Metabolism
229
Chylomicrons, VLDL, and IDL
Chylomicrons and VLDL are primarily triglyceride particles, although they each have small quantities of cholesterol esters.
•Chylomicrons transport dietary triglyceride to adipose tissue and muscle
•VLDL transport triglyceride synthesized in the liver to these same tissues
Both chylomicrons and VLDL have apoC-II, apoE, and apoB (apoB-48 on chylomicrons and apoB-100 on VLDL).
Lipoprotein (LPLase) is required for the metabolism of both chylomicrons and VLDL. This enzyme is induced by insulin and transported to the luminal surface of capillary endothelium, where it is in direct contact with the blood. Lipoprotein lipase hydrolyzes the fatty acids from triglycerides carried by chylomicrons and VLDL and is activated by apoC-II.
INTESTINE (Epithelium)
Cholesterol
LIVER
Triglyceride
Glucose
230
apoB-48 |
|
apoB-48 |
|
|
TGL |
|
TGL |
|
|
ADIPOSE |
CE |
|
CE |
apoC-II |
|
|
Chylomicron |
|
apoE |
|
|
(lymph) |
|
|
|
|
Chylomicron |
|
Lipoprotein |
|
+ |
|
|
(blood) |
|
|
lipase |
apoE |
apoB-48 |
|
Fatty acids |
|
TGL |
|
|
|
|
|
|
|
Glycerol 3-P |
|
CE |
|
|
|
|
Chylomicron |
|
|
|
|
remnant |
apoB-100 |
|
Triglycerides |
TGL apoB-100 |
TGL |
|
|
|
|
chol |
|
chol |
|
|
|
VLDL |
|
apoE apoC-II |
|
Glycerol 3-P |
(blood) |
|
VLDL |
|
|
|
+ |
Lipoprotein |
|
|
(blood) |
lipase |
apoE |
apoB-100 |
|
|
Fatty acids |
|
TGL |
|
|
|
|
|
|
|
|
|
chol |
|
|
|
|
|
IDL |
|
|
|
|
Figure I-15-6. Chylomicron and VLDL Metabolism
Chylomicrons
Chylomicrons are assembled from dietary triglycerides (containing predominantly the longer chain fatty acids, including the essential fatty acids), cholesterol esters, and the 4 lipid-soluble vitamins. The core lipid is surrounded by phospholipids similar to those found in cell membranes, which increase the solubility of chylomicrons in lymph and blood. ApoB-48 is attached and required for release from the epithelial cells into the lymphatics.
Chapter 15 ● Lipid Synthesis and Storage
Chylomicrons leave the lymph and enter the peripheral blood, where the thoracic duct joins the left subclavian vein, thus initially bypassing the liver. After a high-fat meal, chylomicrons cause serum to become turbid or milky. While in the blood, chylomicrons acquire apoC-II and apoE from HDL particles.
In capillaries of adipose tissue (and muscle), apoC-II activates lipoprotein lipase, the fatty acids released enter the tissue for storage, and the glycerol is retrieved by the liver, which has glycerol kinase. The chylomicron remnant is picked up by hepatocytes through the apoE receptor; thus, dietary cholesterol, as well as any remaining triglyceride, is released in the hepatocyte.
VLDL
The metabolism of VLDL is very similar to that of chylomicrons, the major difference being that VLDL are assembled in hepatocytes to transport triglyceride containing fatty acids newly synthesized from excess glucose, or retrieved from the chylomicron remnants, to adipose tissue and muscle. ApoB-100 is added in the hepatocytes to mediate release into the blood. Like chylomicrons, VLDL acquire apoC-II and apoE from HDL in the blood and are metabolized by lipoprotein lipase in adipose tissue and muscle.
VLDL remnants (IDL)
After triglyceride is removed from the VLDL, the resulting particle is called a VLDL remnant or an IDL. A portion of the IDLs is picked up by hepatocytes through their apoE receptor, but some of the IDLs remain in the blood, where they are further metabolized. These IDLs are transition particles between triglyceride and cholesterol transport. In the blood, they can acquire cholesterol esters transferred from HDL particles and thus become converted into LDLs.
Although both LDL and HDL are primarily cholesterol particles, most of the cholesterol measured in the blood is associated with LDL. The normal role of LDL is to deliver cholesterol to tissues for biosynthesis. When a cell is repairing membrane or dividing, the cholesterol is required for membrane synthesis. Bile acids and salts are made from cholesterol in the liver, and many other tissues require some cholesterol for steroid synthesis. About 80% of LDL are picked up by hepatocytes, the remainder by peripheral tissues.
ApoB-100 is the only apoprotein on LDL, and endocytosis of LDL is mediated by apoB-100 receptors (LDL receptors) clustered in areas of cell membranes lined with the protein clathrin.
The liver has multiple pathways for acquiring cholesterol, including:
•De novo synthesis
•Endocytosis of LDL
•Transfer of cholesterol from HDL via the SR-B1 receptor
•Endocytosis of chylomicron remnants with residual dietary cholesterol
231
Part I ● Biochemistry
Increased cholesterol in the hepatocytes inhibits further accumulation by repressing the expression of the genes for HMG-CoA reductase, the LDL receptor, and the SR-B1 receptor.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LDL binds to LDL (apoB-100) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
receptors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hepatocyte |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Endocytosis (clathrin-coated pits) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lysosomal fusion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Receptor recycling |
Release of free cholesterol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
De novo |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
synthesis |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cholesterol |
|
Acetyl-CoA |
|
|
|
|
|
|
|
|
|
|
|
|
|
– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LDL-receptor |
– |
+ |
|
|
|
|
|
|
HMG-CoA |
|
|
|
gene expression |
|
|
|
|
|
|
|
reductase |
|
|
|
|
|
|
|
|
|
|
|
|
ACAT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ACAT: Acyl cholesterol |
Cholesterol |
esters |
acyl transferase |
(storage) |
|
|
|
|
|
|
|
|
|
|
Bile acids |
Figure I-15-7. Regulation of Cholesterol Level in Hepatocytes
Endocytosis involves:
•Formation of a coated pit, which further invaginates to become an endosome
•Fusion of the endosome with a lysosome, accompanied by acidification and activation of lysosomal enzymes
•Release of LDL from the LDL receptor
The receptor may recycle to the surface, the LDL is degraded, and cholesterol is released into the cell. Expression of the gene for LDL receptors (apoB-100 receptor) is regulated by the cholesterol level within the cell. High cholesterol decreases expression of this gene as well as the gene for HMG-CoA reductase, the rate limiting enzyme of de novo cholesterol synthesis.
HDL
HDL is synthesized in the liver and intestines and released as dense, protein-rich particles into the blood. The particles contain apoA-1 used for cholesterol recovery from fatty streaks in the blood vessels. HDL also carry apoE and apoC-II, but those apoproteins are primarily to donate temporarily to chylomicrons and VLDL.
232

Chapter 15 ● Lipid Synthesis and Storage
•Lecithin–cholesterol acyltransferase (LCAT) (or PCAT, phosphatidyl- choline–cholesterol acyltransferase) is an enzyme in the blood that is activated by apoA-1 on HDL.
–LCAT adds a fatty acid to cholesterol, producing cholesterol esters, which dissolve in the core of the HDL.
–This allows HDL to transport cholesterol from the periphery to the liver.
•HDL cholesterol esters picked up in the periphery can be distributed to other lipoprotein particles such as VLDL remnants (IDL), converting them to LDL. The cholesterol ester transfer protein (CETP) facilitates this transfer.
•HDL cholesterol picked up in the periphery can also enter cells through a scavenger receptor, SR-B1.
–This receptor is expressed at high levels in hepatocytes and the steroidogenic tissues, including ovaries, testes, and areas of the adrenal glands.
–This receptor does not mediate endocytosis of the HDL, but rather transfer of cholesterol into the cell by a mechanism not yet clearly defined.
The metabolism of LDL and HDL intersects in the production and control of fatty streaks and potential plaques in blood vessels. The figure below illustrates one model of atherosclerosis involving HDL and LDL at the site of endothelial cell injury. Damage to the endothelium may be related to many factors, including normal turbulence of the blood, elevated LDL, especially modified or oxidized LDL, free radicals from cigarette smoking, homocystinemia (Chapter 17), diabetes (glycation of LDL), and hypertension. The atherosclerotic lesion represents an inflammatory response sharing several characteristics with granuloma formation, and not simple deposition of cholesterol in the blood vessel.
•Endothelial dysfunction increases adhesiveness and permeability of the endothelium for platelets and leukocytes. Infiltrations involve monocytes and T cells. Damaged endothelium has procoagulant rather than anticoagulant properties. In some cases, the endothelial lining may become partially denuded.
•Local inflammation recruits monocytes and macrophages with subsequent production of reactive oxygen species. LDL can become oxidized and then taken up, along with other inflammatory debris, by macrophages, which can become laden with cholesterol (foam cells). Initially the subendothelial accumulation of cholesterol-laden macrophages produces fatty streaks.
•As the fatty streak enlarges over time, necrotic tissue and free lipid accumulates, surrounded by epithelioid cells and eventually smooth muscle cells, an advanced plaque with a fibrous cap. The plaque eventually begins to occlude the blood vessel, causing ischemia and infarction in the heart, brain, or extremities.
233








 Cholesterol
Cholesterol









 3P
3P