Part I ● Biochemistry
Clinical Correlate
In chronic myelogenous leukemia (CML), the presence of the Philadelphia chromosome translocation (t[9;22]) produces a BCR-ABL, an abnormal fusion protein with tyrosine kinase activity. It has been shown that monitoring the level of BCR-ABL mRNA with an RT-PCR in CML patients during therapy with Imatinib (a tyrosine kinase inhibitor) is helpful for both prognosis and management of therapy.
A PCR for the HIV provirus has 2 important advantages over the ELISA/ Western blot:
•Is positive much earlier after infection
•Does not rely on an antibody response by the individual
Important situations in which the PCR is currently used include HIV testing in newborns whose mothers are HIV positive (will always be positive in ELISA/ Western blot) and early testing after known exposure to HIV-positive blood
(e.g., needlesticks) or other fluids/tissue.
Reverse Transcriptase PCR (RT-PCR)
An RT-PCR detects and can quantify a specific RNA rather than DNA in a sample. This test is useful in detecting RNA viruses such as HIV and, in situations similar to the Northern blot, determining whether a gene is transcribed.
Measuring viral load in AIDS patients
The RT-PCR is used to measure the concentration of active circulating virus in the blood of an AIDS patient (viral load). In this way, the test can be used to monitor the status of infection and the infection’s response to antiviral drugs.
Figure I-7-9A shows the steps involved. A blood sample from an HIV-infected individual is obtained and, after appropriate preparation, is treated with reverse transcriptase to produce cDNA from any RNA in the sample. The cDNA is subsequently PCR-amplified using primers specific for the end sequences of the HIV cDNA. The amplified product is quantitated and, with the use of a standard curve (Figure I-7-9B), can be related to the original amount of HIV RNA present.
|
|
|
|
|
PCR with |
|
|
Reverse |
primers |
|
|
specific for |
|
transcriptase |
HIV cDNA |
RNA in blood |
|
|
|
cDNAs reverse |
|
|
PCR-amplified |
|
|
|
transcribed from RNA |
|
|
cDNA from HIV |
sample |
|
|
|
|
|
|
|
|
in blood sample |
|
|
in blood sample |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A
of amplified product |
|
Amount PCR |
|
|
|
|
Concentration of HIV-RNA |
B |
in original sample (copies/ml) |
|
Figure I-7-9. Quantifying Viral Load in HIV Infection Using a Reverse Transcriptase PCR (RT-PCR)
(A) RT-PCR technique (B) Standard curve for quantifying HIV-RNA in blood sample
114
Chapter 7 ● Techniques of Genetic Analysis
Review Questions
1.Two sets of parents were friends in a small town and had babies on the same day. The wristbands of the two similar-looking infants (A and B) were inadvertently mixed at the pediatric care unit. In order to accurately identify the parents of the respective infants, PCR analysis was performed on samples of blood taken from the two infants and both sets of parents (Father 1 and Mother 1 versus Father 2 and Mother 2). Shown below is the analysis of the PCR products by gel electrophoresis.
Parents 1 |
|
|
Parents 2 |
F1 |
M1 |
A |
B |
M2 |
F2 |
|
|
|
|
|
|
What is the best conclusion from the analysis?
A.A is the child of Parents 1.
B.A is the child of Parents 2.
C.B is the child of Parents 1.
D.Father 1 (F1) could be the father of both infants.
E.(Father 2 (F2) could be the father of both infants.
2.Paternal relationship between a man and infant can be best determined by the technique commonly referred to as DNA fingerprinting. Which of the following sequences is most conveniently analyzed in a DNA fingerprint?
A.Histocompatibility loci
B.Centromeres
C.Microsatellite tandem repeats (STRs)
D.Restriction enzyme sites
E.(Single-copy sequences
115
Part I ● Biochemistry
3.Sickle cell anemia is caused by a missense mutation in codon 6 of the β-globin gene.
|
Codon number |
|
5 |
6 |
7 |
8 |
Normal allele |
CCT GAG GAG AAG |
Mutant allele |
CCT GTG GAG AAG |
A man with sickle cell disease and his phenotypically normal wife request genetic testing because they are concerned about the risk for their unborn child. DNA samples from the man and the woman and from fetal cells obtained by amniocentesis are analyzed using the PCR to amplify exon 1 of the β-globin gene. Which 12-base nucleotide sequence was most likely used as a specific probe complementary to the coding strand of the sickle cell allele?
A.CCTCACCTCAGG
B.CCTGTGGAGAAG
C.GGACACCTCTTC
D.CTTCTCCACAGG
E.CTTCTCCTCAGG
4.mRNA encoding glucose 6-phosphatase was isolated from baboon liver and used to make a 32P-cDNA probe. DNA was then isolated from mar-
moset and from human tissue, digested with a restriction endonuclease, Southern blotted, and probed with the 32P-cDNA. Which of the following conclusions can be drawn from the results of this analysis shown below?
A.The glucose 6-phosphatase gene is present in baboon, marmoset and human liver.
B.Both marmoset and human liver express the glucose 6-phosphatase gene.
C.There are two glucose 6-phosphatase genes in the human liver.
D.The glucose 6-phosphatase gene is on different chromosomes in the marmoset and in the human.
E.The human and marmoset tissue used in this experiment is from liver.
116
Chapter 7 ● Techniques of Genetic Analysis
5.A couple seeks genetic counseling because both the man and the woman (unrelated to each other) are carriers of a mutation causing β-thalassemia, an autosomal recessive condition. The couple has one son who is phenotypically normal and has been shown by DNA analysis to be homozygous for the normal allele. They wish to know whether the fetus in the current pregnancy will have β-thalassemia. Using a probe for the β-globin gene that detects a BamHI RFLP, the following results are obtained. What is the best conclusion about the fetus?
A.The fetus has inherited the mutation from both parents.
B.The fetus has inherited the mutation from the mother but not from the father.
C.The fetus has inherited the mutation from the father but not from the mother.
D.The fetus has not inherited the mutation from either parent.
E.The results are inconclusive.
117
Part I ● Biochemistry
Answers
1.Answer: A. Among the conclusions offered, only A is consistent with the results on the blot. Infant A’s pattern shows a PCR product (lower on the blot) matching F1 and another PCR product (higher on the blot) matching M1. Neither of infant A’s PCR products match F2 (choices B and E). The upper PCR product in infant B’s pattern does not match with either F1 or M1 (choices C and D). Although unlikely given the situation, another possibility is consistent with the blot. Infant A could be the child of M2 and F1, although this is not offered as an option.
2.Answer: C. STR sequences are amplified using a PCR and analyzed by gel electrophoresis. Although RFLP analysis could potentially be used for this purpose, it is not the method of choice.
3.Answer: D. The complementary probe will be antiparallel to the coding strand of the mutant allele, with all sequences written 5′ → 3′.
4.Answer: A. All 3 tissues contain the gene (the probe was produced from baboon mRNA, implying the gene is also there).
5.Answer: C. Knowing the son is homozygous for the normal allele, one can conclude that the two restriction fragments shown in his pattern derived from chromosomes without the mutation. It is also clear that the upper (larger) fragment came from his mother’s chromosome and the lower (smaller) fragment came from his father’s chromosome. The fetus has the fragment from his mother’s normal chromosome. The other fragment (top one on the blot) must have come from the father’s chromosome with the mutation. The fetus therefore is heterozygous for the mutation and the normal allele of the β-globin gene.
118
Part I ● Biochemistry
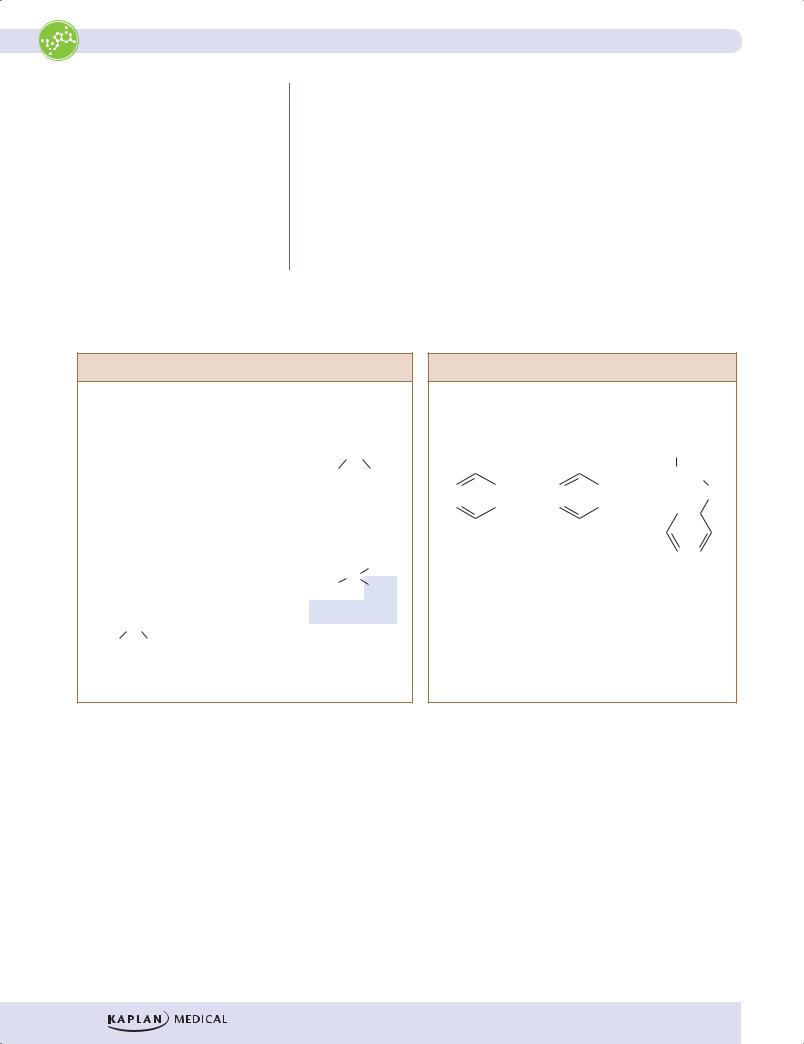
Additional points about some of these amino acids include:
•Serine and threonine are sites for O-linked glycosylation of proteins, a posttranslational modification that should be associated with the Golgi apparatus.
•Asparagine is a site for N-linked glycosylation of proteins, a cotranslational modification that should be associated with the endoplasmic reticulum.
•Cysteine contains sulfur and can form disulfide bonds to stabilize the shape (tertiary structure) of proteins. Destroying disulfide bonds denatures proteins.
•Methionine, another sulfur-containing amino acid, is part of S-adenosylmethionine (SAM), a methyl donor in biochemical pathways.
Nonpolar, Aliphatic Side Chains
; |
|
COO: |
; |
|
|
|
COO: |
; |
|
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
H |
H3N |
|
|
|
C |
|
|
H |
|
H3N |
|
|
C |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
CH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
CH3 |
|
Glycine |
|
|
|
Alanine |
|
|
|
|
Valine |
|
|
Gly |
|
|
|
|
|
|
Ala |
|
|
|
|
|
|
Val |
|
|
|
|
|
COO: |
|
|
|
|
|
|
COO: |
|
|
|
|
|
|
COO: |
; |
|
|
|
|
|
; |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
H3N |
|
C |
|
H |
H3N |
|
|
|
C |
|
|
|
H |
; |
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
2N |
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
H |
|
|
|
|
C |
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2C |
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
CH3 |
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
Leucine |
|
Isoleucine |
|
|
|
|
Proline |
|
|
|
Leu |
|
|
|
|
|
|
|
Ile |
|
|
|
|
|
|
Pro |
Aromatic Side Chains
; |
|
COO: |
; |
|
COO: |
; |
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
H |
H3N |
|
C |
|
H |
H3N |
|
C |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
CH2 |
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
CH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phenylalanine |
|
Tyrosine |
|
Tryptophan |
|
|
|
Phe |
|
|
|
Tyr |
|
|
|
|
|
|
Trp |
Figure I-8-2. The Hydrophobic Amino Acids
Note: Tyrosine can be considered nonpolar or polar because of the ability of the -OH group to form a hydrogen bond.
120
Chapter 8 ● Amino Acids, Proteins, and Enzymes
Positively Charged R Groups
; |
|
|
COO: |
|
; |
|
|
COO: |
; |
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
|
H |
H3N |
|
C |
|
H |
H3N |
|
C |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
NH |
|
|
|
CH2 |
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
; CH |
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
NH |
|
|
|
|
|
|
|
|
|
C |
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
H |
|
|
|
|
|
NH3; |
|
|
|
|
|
|
|
|
|
|
|
NH2; |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lysine |
|
|
|
NH2 |
|
|
|
|
|
|
|
|
Histidine |
|
|
Arginine |
|
|
|
|
|
|
|
|
Lys |
|
|
|
Arg |
|
|
|
|
|
|
|
His |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Negatively Charged R Groups |
|
|
|
|
; |
|
|
|
COO: |
; |
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
H |
|
|
|
|
|
|
|
|
H3N |
|
|
C |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aspartate |
|
|
|
|
|
|
|
|
Glutamate |
|
|
|
|
|
|
|
|
|
|
|
|
Asp |
|
|
|
|
|
|
|
|
|
|
|
|
Glu |
|
|
|
|
Polar, Uncharged R Groups
; |
|
|
COO: |
; |
|
|
|
|
COO: |
; |
|
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
H |
H3N |
|
C |
|
H |
H3N |
|
C |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
C |
|
|
OH |
|
|
|
|
CH2 |
|
|
|
|
CH2OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
SH |
|
Serine |
|
|
Threonine |
|
Cysteine |
|
|
|
Ser |
|
|
|
|
|
|
Thr |
|
|
Cys |
|
|
|
|
; |
|
|
COO: |
; |
|
|
|
COO: |
; |
|
COO: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3N |
|
C |
|
|
H |
H3N |
|
|
C |
|
|
H |
H3N |
|
C |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
H2N |
|
|
|
|
O |
|
|
|
|
|
C |
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2N |
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asparagine |
|
Glutamine |
Methionine |
|
|
|
Met |
|
|
|
|
Asn |
|
|
Gln |
|
|
|
|
Figure I-8-3. The Hydrophilic Amino Acids
Note: Methionine can be considered nonpolar or polar because it contains a sulfur.
121
Part I ● Biochemistry
Hemoglobinopathy
An 8-year-old African American boy was experiencing pain in the chest and back. He was taken to the hospital, where he was found to have mild anemia, splenomegaly, and rod-shaped crystals in the erythrocytes. A preliminary diagnosis of sickle cell anemia was made. To validate the diagnosis, a small aliquot of his blood was subjected to electrophoresis to determine the identity of the hemoglobin in his erythrocytes. After reviewing the data, the physician concluded that he did not have sickle cell anemia, but rather a sickle cell anemia– like hemoglobinopathy with the relatively common mutation of HbC.
|
|
|
dar |
d |
|
|
|
n |
t |
|
|
n |
|
|
|
ie |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
St |
a |
|
|
|
P |
a |
t |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HbA
HbS
HbC
Sickle cell anemia is characterized by severe pain in the bones, abdomen, and chest, along with periods of hemolytic problems. Episodes of vasoocclusive pain lasting approximately 1 week are a frequent problem. These crises are often precipitated by dehydration or infection. A widely used method to analyze hemoglobins found in various hemoglobinopathies is electrophoresis at pH 8.4, where single amino acid substitutions can be easily detected. In sickle cell anemia, there is a substitution of valine for glutamate at position 6 in Hb, meaning that the HbS will have one less negative charge overall compared with HbA. In HbC, there is a substitution of lysine for glutamate at position 6, meaning that HbC will have two additional positive charges compared with HbA. These 3 hemoglobins can be resolved by electrophoresis.
PROTEIN TURNOVER AND AMINO ACID NUTRITION
When older proteins are broken down in the body, they must be replaced. This concept is called protein turnover, and different types of proteins have very different turnover rates. Protein synthesis occurs during the process of translation on ribosomes. Protein breakdown tends to occurs in 2 cellular locations:
•Lysosomal proteases digest endocytosed proteins.
•Large cytoplasmic complexes (or proteasomes) digest older or abnormal proteins that have been covalently tagged with a protein (called ubiquitin) for destruction.
122
Chapter 8 ● Amino Acids, Proteins, and Enzymes
Essential Amino Acids
All 20 types of amino acids are required for protein synthesis. These amino acids can be derived from digesting dietary protein and absorbing their constituent amino acids or, alternatively, by synthesizing them de novo.
There are 10 amino acids that cannot be synthesized in humans and thus must be provided from dietary sources. These are called the essential amino acids.
Arginine is required only during periods of growth or positive nitrogen balance.
Table I-8-1. Essential Amino Acids
Arginine* |
Methionine |
|
|
Histidine |
Phenylalanine |
|
|
Isoleucine |
Threonine |
|
|
Leucine |
Tryptophan |
|
|
Lysine |
Valine |
|
|
*Essential only during periods of positive nitrogen balance.
Nitrogen Balance
Nitrogen balance is the (normal) condition in which the amount of nitrogen incorporated into the body each day exactly equals the amount excreted.
Negative nitrogen balance occurs when nitrogen loss exceeds incorporation. It is associated with:
•Protein malnutrition (kwashiorkor)
•Dietary deficiency of even 1 essential amino acid
•Starvation
•Uncontrolled diabetes
•Infection
Positive nitrogen balance occurs when the amount of nitrogen incorporated exceeds the amount excreted. It is associated with:
•Growth
•Pregnancy
•Convalescence (recovery phase of injury or surgery)
•Recovery from condition associated with negative nitrogen balance
Note
Do not confuse kwashiorkor with marasmus. Marasmus is a chronic deficiency of calories; patients do not present with edema as they do with kwashiorkor.