
Erik_Kandel_-_V_poiskakh_pamyati_angl
.pdf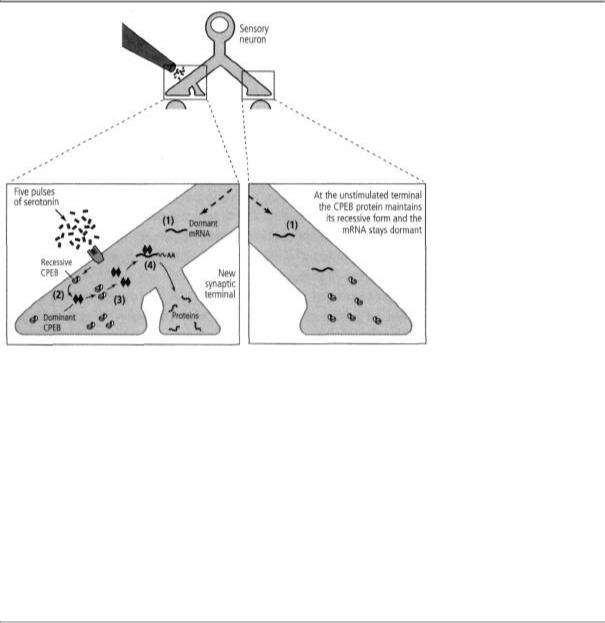
A wild idea! But if true, it could explain how long-term memory is maintained in synapses indefinitely, despite constant protein degradation and turnover. Clearly, a self-perpetuating molecule could remain at a synapse indefinitely, regulating the local protein synthesis needed to maintain newly grown synaptic terminals.
In my late-night thoughts about long-term memory, I had once briefly entertained the idea that prions might somehow be involved in long-term memory storage. Moreover, I was familiar with Prusiner's groundbreaking work on prions and prion diseases, work for which he would receive the 1997 Nobel Prize in Medicine or Physiology. Thus, while I had never anticipated that the novel form of CPEB might be a prion, I was immediately enthusiastic about Kausik's ideas.
Prions were a major field of study in yeast, but no one had identified a normal function of these proteins until Kausik's discovery of the novel form of CPEB in neurons. Thus his discovery not only offered deep new insights into learning and memory, it broke new ground in biology We soon found that in the sensory neurons of the gillwithdrawal reflex, the conversion of CPEB from the inactive, non-
19-4 Long-term memory and the prion-like CPEB protein. As a result of a prior stimulus, the sensory cell's nucleus has sent dormant messenger RNA (mRNA) to all axon terminals (1). Five pulses of serotonin at one terminal convert a prion-like protein (CPEB) that is present at all synapses into a dominant, self-perpetuating form (2). Dominant CPEB can convert recessive CPEBs to the dominant form (3). Dominant CPEB activates dormant messenger RNA (4). The activated messenger RNA regulates protein synthesis at the new synaptic terminal, stabilizes the synapse, and perpetuates the memory.
propagating form to the active, propagating form is controlled by serotonin, the transmitter that is required for converting shortto long-term memory (figure 19-4). In its self-perpetuating form, CPEB maintains local protein synthesis. Moreover, the self-perpetuating state is not easily reversed.
These two features make the new variant of the prion ideally designed for memory storage. Selfperpetuation of a protein that is critical for local protein synthesis allows information to be stored selectively and in perpetuity at one synapse, and not, Kausik soon discovered, at the many others that a neuron makes with its target cells.
Beyond discovering the new prion's relevance to the persistence of memory or even to the functioning of the brain, Kausik and I had found two new biological features of prions. First, a normal

physiological signal—serotonin—is critical for converting CPEB from one form to another. Second, CPEB is the first self-propagating form of a prion known to serve a physiological function—in this case, perpetuation of synaptic facilitation and memory storage. In all other cases previously studied, the self-propagating form either causes disease and death by killing nerve cells or, more rarely, is inactive.
We have come to believe that Kausik's discovery may be just the tip of a new biological iceberg. In principle, this mechanism—activation of a nonheritable, self-perpetuating change in a protein—could operate in many other biological contexts, including development and gene transcription.
This exciting finding in my laboratory illustrates that basic science can be like a good mystery novel with surprising twists: some new, astonishing process lurks in an undiscovered corner of life and is later found to have wide significance. This particular finding was unusual in that the molecular processes underlying a group of strange brain diseases are now seen also to underlie long-term memory, a fundamental aspect of healthy brain function. Usually, basic biology contributes to our understanding of disease states, not vice versa.
IN RETROSPECT, OUR WORK ON LONG-TERM SENSITIZATION AND
the discovery of the prionlike mechanism brought to the forefront three new principles that relate not only to Aplysia but to memory storage in all animals, including people. First, activating long-term memory requires the switching on of genes. Second, there is a biological constraint on what experiences get stored in memory. To switch on the genes for long-term memory, CREB-1 proteins must be activated and CREB-2 proteins, which suppress the memory-enhancing genes, must be inactivated. Since people do not remember everything they have learned—nor would anyone want to—it is clear that the genes that encode suppressor proteins set a high threshold for converting shortterm to long-term memory. It is for this reason that we remember only certain events and experiences for the long run. Most
things we simply forget. Removing that biological constraint triggers the switch to long-term memory. The genes activated by CREB-1 are required for new synaptic growth. The fact that a gene must be switched on to form long-term memory shows clearly that genes are not simply determinants of behavior but are also responsive to environmental stimulation, such as learning.
Finally, the growth and maintenance of new synaptic terminals makes memory persist. Thus, if you remember anything of this book, it will be because your brain is slightly different after you have finished reading it. This ability to grow new synaptic connections as a result of experience appears to have been conserved throughout evolution. As an example, in people, as in simpler animals, the cortical maps of the body surface are subject to constant modification in response to changing input from sensory pathways.
FOUR
These scenes. . . . why do they survive undamaged year after year unless they are made of something comparatively permanent? —Virginia Woolf, "Sketch of the Past" (1953)
20

A RETURN TO COMPLEX MEMORY
When I first began to study the biological basis of memory, I focused on the memory storage that ensues from the three simplest forms of learning: habituation, sensitization, and classical conditioning. I found that when a simple motor behavior is modified by learning, those modifications directly affect the neural circuit responsible for the behavior, altering the strength of preexisting connections. Once stored in the neural circuit, that memory can be recalled immediately.
This finding gave us our first insight into the biology of implicit memory, a form of memory that is not recalled consciously. Implicit memory is responsible not only for simple perceptual and motor skills but also, in principle, for the pirouettes of Margot Fonteyn, the trumpeting technique of Wynton Marsalis, the accurate ground strokes of Andre Agassi, and the leg movements of an adolescent riding a bicycle. Implicit memory guides us through well-established routines that are not consciously controlled.
The more complex memory that had inspired me initially—the explicit memory for people, objects, and places— is consciously recalled and can typically be expressed in images or words. Explicit memory is far more sophisticated than the simple reflex I had studied
in Aplysia. It depends on the elaborate neural circuitry of the hippocampus and the medial temporal lobe, and it has many more possible storage sites.
Explicit memory is highly individual. Some people live with such memories all the time. Virginia Woolf falls into this category. Her memories of childhood were always at the edge of her consciousness, ready to be summoned up and incorporated into everyday moments, and she had an exquisite ability to describe the details of her recalled experiences. Thus, years after the death of her mother, Woolf's memory of her was still fresh:
. . . there she was, in the very center of that great Cathedral space which was childhood; there she was from the very first. My first memory is of her lap. . . . Then I see her in her white dressing gown on the balcony. ... It is perfectly true that she obsessed me in spite of the fact that she died when I was thirteen, until I was forty-four.
. . . these scenes . . . why do they survive undamaged year after year unless they are made of something comparatively permanent?
Other people call up their past life only occasionally. Periodically, I think back and recall the two police officers coming to our apartment and ordering us to leave on the day of Kristallnacht. When this memory enters my consciousness, I can once again see and feel their presence. I can visualize the worried expression on my mother's face, feel the anxiety in my body, and perceive the confidence in my brother's actions while retrieving his coin and stamp collections. Once I place these memories in the context of the spatial layout of our small apartment, the remaining details emerge in my mind with surprising clarity.
Remembering such details of an event is like recalling a dream or watching a movie in which we play a part. We can even recall past emotional states, though often in a much simplified form. To this day I remember some of the emotional context of my romantic encounter with our housekeeper Mitzi.
As Tennessee Williams wrote in The Milk Train Doesn't Stop Here Anymore, describing what we now call explicit memory, "Has it ever struck you . . . that life is all memory, except for the one present moment that goes by you so quickly you hardly catch it going? It's really all memory . . . except for each passing moment."
For all of us, explicit memory makes it possible to leap across space and time and conjure up events and emotional states that have vanished into the past yet somehow continue to live on in our minds. But recalling a memory episodically—no matter how important the memory—is not like simply turning to a photograph in an album. Recall of memory is a creative process. What the brain stores is thought to be only a core memory. Upon

recall, this core memory is then elaborated upon and reconstructed, with subtractions, additions, elaborations, and distortions. What biological processes enable me to review my own history with such emotional vividness?
ON REACHING MY SIXTIETH BIRTHDAY, I FINALLY GATHERED THE
courage to return to the study of the hippocampus and explicit memory. I had long been curious to see whether some of the basic molecular principles we had learned from a simple reflex circuit in Aplysia also applied to the complex neural circuits of the mammalian brain. By 1989, three major breakthroughs had made it feasible to explore this question in the laboratory.
The first was the discovery that the pyramidal cells of the hippocampus play a critical role in an animal's perception of its spatial environment. The second was the discovery of a remarkable synaptic strengthening mechanism in the hippocampus called long-term potentiation. Many researchers thought this mechanism might underlie explicit memory. The third breakthrough, and the one most immediately relevant to my own molecular approach to learning, was the invention of powerful new methodologies for modifying mice genetically. My colleagues and I would adapt those methods to the brain in an attempt to explore explicit memory in the hippocampus in the same molecular detail as we had studied implicit memory in Aplysia.
The new era of research in the hippocampus began in 1971, when
John O'Keefe at University College, London made an amazing discovery about how the hippocampus processes sensory information. He found that neurons in the hippocampus of the rat register information not about a single sensory modality—sight, sound, touch, or pain— but about the space surrounding the animal, a modality that depends on information from several senses. He went on to show that the hippocampus of rats contains a representation—a map—of external space and that the units of that map are the pyramidal cells of the hippocampus, which process information about place. In fact, the pattern of action potentials in these neurons is so distinctively related to a particular area of space that O'Keefe referred to them as "place cells." Soon after O'Keefe's discovery, experiments with rodents showed that damage to the hippocampus severely compromises the animals' ability to learn a task that relies on spatial information. This finding indicated that the spatial map plays a central role in spatial cognition, our awareness of the environment around us.
Since space involves information acquired through several sensory modalities, it raised the questions: How are these modalities brought together? How is the spatial map established? Once established, how is the spatial map maintained?
The first clue to the answers emerged in 1973, when Terje Lomo and Tim Bliss, postdoctoral students in Per Andersen's laboratory in Oslo, discovered that the neuronal pathways leading to the hippocampus of rabbits can be strengthened by a brief burst of neural activity. Lomo and Bliss were unaware of O'Keefe's work and did not attempt to examine the functioning of the hippocampus in the context of memory or a specific behavior, as we had done with Aplysias gill-withdrawal reflex. Instead, they adopted an approach similar to the one Ladislav Tauc and I had first taken in 1962: they developed a neural analog of learning. Rather than basing their neural analog on conventional behavioral paradigms, such as habituation, sensitization, or classical conditioning, they based it on neuronal activity per se. They applied a very rapid train of electrical stimuli (100 impulses per second) to a neuronal pathway leading to the hippocampus and found that the synaptic connections in that pathway were strengthened for
several hours to one or more days. Lomo and Bliss called this form of synaptic facilitation long-term potentiation.

It soon emerged that long-term potentiation occurs in all three of the pathways within the hippocampus and that it is not a unitary process. Instead, long-term potentiation describes a family of slightly different mechanisms, each of which increases the strength of the synapse in response to different rates and patterns of stimulation. Long-term potentiation is analogous to long-term facilitation of the connections between sensory and motor neurons in Aplysia in that it enhances the strength of synaptic connections. But whereas long-term facilitation in Aplysia strengthens synapses heterosynaptically, by means of a modulatory transmitter acting on the homosynaptic pathway, many of long-term potentiation can be initiated merely by means of homosynaptic activity. As we and others found later, however, neuromodulators are usually recruited to switch short-term homosynaptic plasticity into long-term heterosynaptic plasticity.
In the early 1980s, Andersen simplified Lomo and Bliss's research methodology greatly by removing the hippocampus from the brain of a rat, cutting it into slices, and placing the slices in an experimental dish. This enabled him to observe the several neural pathways in a specific segment of the hippocampus. Amazingly, such brain slices can function for hours when prepared properly. With this advance, researchers could analyze the biochemistry of long-term potentiation and observe the effects of drugs blocking various signaling components.
Key molecules involved in long-term potentiation began to emerge from such studies. In the 1960s David Curtis collaborating with Geoffrey Watkins discovered that glutamate, a common amino acid, is the major excitatory transmitter in the vertebrate brain (just as it is in the invertebrate brain, we later discovered). Watkins and Graham Collingridge then found that glutamate acts on two different types of ionotropic receptors in the hippocampus, the AMPA receptor and the NMDA receptor. The AMPA receptor mediates normal synaptic transmission and responds to an individual action potential in the presynaptic neuron. The NMDA receptor, on the other hand, responds only to
extraordinarily rapid trains of stimuli and is required for long-term potentiation.
When a postsynaptic neuron is stimulated repeatedly, as in Bliss and Lomo's experiments, the AMPA receptor generates a powerful synaptic potential that depolarizes the cell membrane by as much as 20 or 30 millivolts. This depolarization causes an ion channel in the NMDA receptor to open, allowing calcium to flow into the cell. Roger Nicoll at the University of California, San Francisco and Gary Lynch at the University of California, Irvine discovered independendy that the flow of calcium ions into the postsynaptic cell acts as a second messenger (much as cyclic AMP does), triggering long-term potentiation. Thus the NMDA receptor can translate the electrical signal of the synaptic potential into a biochemical signal.
These biochemical reactions are important because they trigger molecular signals that can be broadcast throughout the cell and thus contribute to long-lasting synaptic modifications. Specifically, calcium activates a kinase (called the calcium, calmodulin-dependent protein kinase) that increases synaptic strength for about an hour. Nicoll went on to show that the calcium influx and the activation of this kinase lead to the strengthening of synaptic connections by causing additional AMPA receptors to be assembled and inserted into the membrane of the postsynaptic cell.
The analysis of how the NMDA receptor functions elicited great excitement among neuroscientists, for it showed that the receptor acts as a coincidence detector. It allows calcium ions to flow through its channel if and only if it detects the coincidence of two neural events, one presynaptic and the other postsynaptic. The presynaptic neuron must be active and release glutamate, and the AMPA receptor in the postsynaptic cell must bind glutamate and depolarize the cell. Only then will the NMDA receptors become active and allow calcium to flow into the cell, thereby triggering long-term potentiation. Interestingly, in 1949 the psychologist D. O. Hebb had predicted that some kind of neural coincidence detector would be present in the brain during learning: "When an axon of cell A . .. excites cell and repeatedly or persistently takes part in its firing, some growth process or metabolic changes take place in one or both cells so that As efficiency is increased."

Aristotle, and subsequently the British empiricist philosophers and many other thinkers, had proposed that learning and memory are somehow the result of mind's ability to associate and form some lasting mental connection between two ideas or stimuli. With the discovery of the NMDA receptor and longterm potentiation, neuro-scientists had unearthed a molecular and cellular process that could well carry out this associative process.
21
SYNAPSES ALSO HOLD OUR FONDEST MEMORIES
The new discoveries in the hippocampus—place cells, the NMDA receptor, and long-term potentiation—raised exciting prospects for neuroscience. But it was not at all clear how the spatial map and long-term potentiation were related to each other or to explicit memory storage. To begin with, although long-term potentiation in the hippocampus was a fascinating and widespread phenomenon, it was a highly artificial way of producing changes in synaptic strength. This artificiality caused even Lomo and Bliss to wonder "whether or not the intact animal makes use in real life of a property which has been revealed by synchronous, repetitive volleys. . . ." Indeed, it seemed unlikely that the same pattern of firing occurs in the course of learning. Many scientists questioned whether the changes in synaptic strength produced by long-term potentiation play any role at all in spatial memory or the formation and maintenance of the spatial map.
I began to realize that the ideal way to explore these relationships would be through genetics, much as Seymour Benzer had used genetics to study learning in Drosophila. In the 1980s biologists began to combine selective breeding and the tools of recombinant DNA to produce genetically modified mice. These techniques made it possible to
manipulate the genes underlying long-term potentiation and thus to answer some pressing questions that interested me. Does long-term potentiation, like long-term facilitation in Aplysia, have different phases? Do those phases correspond to shortand long-term storage of spatial memory? If they do correspond, we could interfere with one or the other phase of long-term potentiation and thereby determine what actually happens to the spatial map in the hippocampus when an animal learns and remembers a new environment.
It was exhilarating for me to return to the hippocampus, an old love found again. I had kept up with the advances in research, so it did not seem as though thirty years had passed. Per Andersen was a good friend, as was Roger Nicoll. But most of all I was motivated by memories of my experiments with Alden Spencer when we were both at NIH. I was feeling once again the excitement of being on the edge of something new—but this time armed with molecular genetic techniques whose power and specificity Alden and I could not have imagined in our wildest dreams.
THESE ADVANCES IN MOLECULAR GENETICS HAD THEIR
intellectual roots in the selective breeding of mice. Experiments at the turn of the twentieth century showed that various lines of mice differ not only in their genetic makeup but also in their behavior. Some lines proved extremely gifted at learning a variety of tasks, while others were exceptionally dull at those tasks. Such observations showed that genes contribute to learning. Animals differ similarly in their degree of fearfulness, sociability, and parenting ability. By inbreeding and creating some lines that are abnormally fearful and others that are not, behavioral geneticists overcame the randomness of natural selection. Selective breeding was thus the first step in isolating the genes responsible for

particular behaviors. Recombinant DNA now made it possible both to try to identify the specific genes needed and to examine the role of those genes in the alteration of the synapses that underlie each behavior, emotional state, or learning capability.
Until 1980 molecular genetics in the mouse relied on a classical analysis known as forward genetics, which is the technique Benzer used in Drosophila. It begins by exposing mice to a chemical that usu-
ally damages only one of the 15,000 genes in the animal's genome. The damage occurs at random, however, so which gene is affected is anyone's guess. The animals are given a variety of tasks to see which, if any, are affected by the randomly altered gene. Because mice must be bred for several generations, forward genetics is very demanding and time-consuming, but it has the great advantage of being unbiased. There are no hypotheses and therefore no biases involved in screening for genes in this manner.
The recombinant DNA revolution enabled molecular biologists to develop a less demanding, less timeconsuming strategy, reverse genetics. In reverse genetics, a specific gene is either removed from or introduced into the mouse's genome and the effects on synaptic change and learning examined. Reverse genetics is biased— it is designed to test a specific hypothesis, such as whether a particular gene and the protein it encodes are involved in a particular behavior.
Two methods of modifying individual genes made reverse genetics in mice possible. The first, transgenesis, involves the introduction of a foreign gene, called a transgene, into the DNA of a mouse egg. Once the egg is fertilized, the transgene becomes part of the genome of the baby mouse. Adult transgenic mice are then bred to obtain a genetically pure strain of mice, all of which express the transgene. The second method of genetically modifying mice involves "knocking out" one gene in the mouse genome. This is done by inserting a segment of genetic material into the mouse's DNA that renders the chosen gene dysfunctional and thus eliminates the protein encoded by that gene from the mouse's body.
IT WAS BECOMING EVIDENT TO ME THAT WITH THESE ADVANCES
in genetic engineering, the mouse would be a superb experimental animal for identifying the genes and proteins responsible for the various forms of long-term potentiation. One could then relate those genes and proteins to the storage of spatial memory. Although mice are relatively simple mammals, they have a brain that is anatomically similar to that of humans and, as in humans, the hippocampus is involved in storing memories of places and objects. Moreover, mice breed much faster than
larger mammals such as cats, dogs, monkeys, and people. As a result, large populations with identical genes, including identical transgenes or knockout genes, can be bred within months.
These revolutionary new experimental techniques also had major biomedical ramifications. Almost every gene in the human genome exists in several different versions, called alleles, which are present in different members of the human population. Genetic studies of human neurological and psychiatric disorders had made it possible to identify alleles that account for behavioral differences in normal people as well as alleles that underlie many neurological disorders, such as amyotrophic lateral sclerosis, early onset Alzheimer's disease, Parkinson's disease, Huntington's disease, and several forms of epilepsy. The ability to insert disease-causing alleles into the mouse genome and then study how they wreak havoc on the brain and on behavior revolutionized neurology.
The final stimulus for me to move into studying genetically engineered mice was the presence in our lab of several talented postdoctoral fellows, among them Seth Grant and Mark Mayford. Grant and Mayford knew mouse genetics much better than I, and they greatly influenced the direction of our research. Grant was the driving force behind my beginning to work on genetically modified mice, while Mayford's critical thinking became important later, as we began to improve on the methodology we and others had used in our first generation of behavioral studies in mice.

Our original methods for producing transgenic mice affected every cell in the mouse's body. We needed to find a way to restrict our genetic manipulations to the brain, specifically to the regions that form the neural circuits of explicit memory. Mayford developed ways of limiting the expression of newly implanted genes to certain regions of the brain. He also developed a method for controlling the timing of gene expression in the brain, thereby making it possible to turn the gene on and off. These two feats inaugurated a new stage in our studies and were widely adopted by other researchers; they remain cornerstones of the modern analysis of behavior in genetically modified mice.
THE FIRST ATTEMPT TO RELATE LONG-TERM POTENTIATION TO
spatial memory was made in the late 1980s. Richard Morris, a physiologist at the University of Edinburgh, had shown that by blocking the NMDA receptor pharmacologically, one could block longterm potentiation and thus interfere with spatial memory. In independent experiments, Grant and I at Columbia and Susumu Tonegawa and his postdoctoral fellow Alcino Silva at the Massachusetts Institute of Technology carried this analysis one important step further. We each created a different line of genetically modified mice that lacked a key protein thought to be involved in long-term potentiation. We then observed how learning and memory were affected in the genetically modified mice, compared with normal mice.
We tested the animals' performance on several well-established spatial tasks. For example, we placed a mouse in the center of a large, white, well-illuminated circular platform surrounded by a rim of forty holes. Only one of the holes led to an escape chamber. The platform was in a small room, each of whose walls was decorated with a different, distinctive marking. Mice do not like being in an open space, especially a well lit one. They feel defenseless and try to escape. The only way a mouse could escape from the platform was to find the hole that led to the escape chamber. Ultimately, the mouse found that hole by learning the spatial relationship between the hole and the markings on the wall.
In trying to escape, mice use three strategies in sequence: random, serial, and spatial. Each strategy allows the animals to find the escape hatch, but each varies greatly in efficiency. The mice first go to any hole at random and quickly learn that this strategy is not efficient. Next, they begin with one hole and then try each consecutive hole until they find the right one. This is a better strategy but still not optimal. Neither strategy is spatial—neither requires the mice to have an internal map of the spatial organization of the environment stored in their brain—and neither strategy requires the hippocampus. Finally, the mice use a spatial strategy that does require the hippocampus. They learn to look to see which marked wall is aligned with the target hole, and then they make a beeline for that hole, using the marking on the wall as a guide. Most mice go
through the first two strategies quickly and soon learn to use the spatial strategy.
We then focused on long-term potentiation in an area of the hippocampus called the Schaffer collateral pathway. Larry Squire of the University of California, San Diego had shown that damage to this one pathway produces a memory deficit similar to that experienced by H.M., Brenda Milner's patient. We found that by knocking out a particular gene encoding a protein that is important for long-term potentiation, we could compromise synaptic strengthening in the Schaffer collateral pathway. Moreover, the genetic defect was correlated with a defect in the mouse's spatial memory.
Each year the Cold Spring Harbor Laboratory holds a meeting devoted solely to one major topic in biology. The topic for 1992 was "The Cell Surface," but because Susumu Tonegawa's and our work relating genes to memory in the mouse was deemed so interesting, a new slot, unrelated to the cell surface, was created for the two of us so we could give back-to-back talks. Tonegawa and I presented our separate experiments on how knocking out a single gene inhibits both long-term potentiation in a pathway of the hippocampus and spatial memory. At the time, it was the most direct correlation that had been made between long-term potentiation and spatial memory.

Soon thereafter, both of us went one step further and examined how long-term potentiation relates to the spatial map of the external environment represented in the hippocampus.
By the time of that meeting, Tonegawa and I already knew each other a bit. In the 1970s he had determined the genetic basis of antibody diversity, an extraordinary contribution to immunology for which he received the Nobel Prize in Physiology or Medicine in 1987. With this accomplishment behind him, he wanted to turn to the brain for new scientific worlds to conquer. He was a good friend of Richard Axel, who suggested that he talk to me.
The problem that most interested Tonegawa when he came to see me in 1987 was consciousness. I tried to encourage his enthusiasm for brain research while dissuading him from taking on consciousness, which was too difficult and too poorly defined for a molecular
approach at that time. Susumu had started to use genetically modified mice to study the immune system, so it was natural and much more realistic for him to turn to learning and memory, which he did when Silva joined his lab.
Since 1992 many other research groups have obtained results parallel to our own. Although there are occasional, important exceptions to the link between disrupted long-term potentiation and deficient spatial memory it has nevertheless proven to be a good place to start examining the molecular mechanisms of long-term potentiation and the role of these molecules in memory storage.
I KNEW THAT SPATIAL MEMORY IN MICE, LIKE THE IMPLICIT
memory studied in Aplysia and Drosophila, has two components: a short-term memory that does not require protein synthesis and a long-term memory that does. Now I wanted to find out whether storage of explicit shortand long-term memory also has distinctive synaptic and molecular mechanisms. In Aplysia, short-term memory requires short-term synaptic changes that rely only on second-messenger signaling. Long-term memory requires more persistent synaptic changes that are based on alterations in gene expression as well.
My colleagues and I examined slices of the hippocampus taken from genetically modified mice and found that in each of the three major pathways of the hippocampus, long-term potentiation has two phases similar to those of long-term facilitation in Aplysia. A single train of electrical stimuli produces a transient, early phase of long-term potentiation that lasts only one to three hours and does not require the synthesis of new protein. The reaction of neurons to those stimuli was just as Roger Nicoll had described: NMDA receptors in the postsynaptic cell are activated, leading to the flow of calcium ions into the postsynaptic cell. Here calcium acts as a second messenger; it triggers long-term potentiation by enhancing the existing AMPA receptors' response to glutamate and by stimulating the insertion of new AMPA receptors into the membrane of the postsynaptic cell. In response to certain patterns of stimulation, the postsynaptic cell also sends a signal back to the presynaptic cell, calling for more glutamate.
Repeated trains of electrical stimuli produce a late phase of long-
term potentiation that lasts for more than a day. We found the properties of this phase, which previously had not been extensively explored, to be very similar to long-term facilitation of synaptic strength in Aplysia. In both Aplysia and mice, the late phase of long-term potentiation is strongly affected by modulatory interneurons, which in mice are recruited to switch a short-term, homosynaptic into long-term, het-erosynaptic change. In mice those neurons release dopamine, a neurotransmitter commonly recruited in the mammalian brain for attention and reinforcement. Like serotonin in

Aplysia, dopamine prompts a receptor in the hippocampus to activate an enzyme that increases the amount of cyclic AMP. However, an important part of the increase in cyclic AMP in the mouse hippocampus occurs in the postsynaptic cell, whereas in Aplysia the increase occurs in the presynaptic sensory neuron. In each case, the cyclic AMP recruits protein kinase A and other protein kinases, which leads to the activation of CREB and the turning on of effector genes.
One of the striking things we had found in studying memory in Aplysia was the existence of the memory-suppressor gene that produces the CREB-2 protein. Blocking the expression of that gene in Aplysia enhances both the strengthening and the increase in number of synapses associated with longterm facilitation. In the mouse, we found that blocking this and similar memory-suppressor genes enhances both long-term potentiation in the hippocampus and spatial memory.
In the course of these studies, I found myself once again in an enjoyable collaboration with Steven Siegelbaum. We were interested in a particular ion channel that inhibits synaptic strengthening, especially in certain dendrites. Alden Spencer and I had studied those dendrites in 1959 and inferred that they produce action potentials in response to activity in the perforant pathway, which leads from the entorrhinal cortex to the hippocampus. Steve and I bred mice in which the gene for this particular ion channel was lacking. We found that long-term potentiation in response to stimulation of the perforant pathway was greatly enhanced in those mice, in part by dendritic action potentials. As a result, these mice were brilliant; they had a much stronger spatial memory than normal mice!
My colleagues and I also discovered that explicit memory in the mammalian brain, unlike implicit memory in Aplysia or Drosophila, requires several gene regulators in addition to CREB. Although the evidence is less complete, it appears that in mice, expression of genes also gives rise to anatomical changes—specifically, to the growth of new synaptic connections.
Despite the significant behavioral differences between implicit and explicit memory, some aspects of implicit memory storage in invertebrates have been conserved over millions of years of evolutionary time in the mechanisms by which explicit memory is stored in vertebrates. Although the great neurophysiologist John Eccles had urged me early in my career not to abandon research on the splendid mammalian brain for work on a slimy, brainless sea snail, it is now clear that several key molecular mechanisms of memory are shared by all animals.
22
THE BRAIN'S PICTURE OF THE EXTERNAL WORLD
The study of the explicit memory for space in the mouse drew me ineluctably to the larger questions that had attracted me to psychoanalysis at the beginning of my career. I was starting to think about the nature of attention and consciousness, mental states not associated with simple reflex actions but with complex psychological processes. I wanted to focus on how space—the internal environment in which the mouse navigates—is represented in the brain and how this representation is modified by attention. In doing so, I was moving from a system in Aplysia that was reasonably well understood to systems in the mammalian brain that had yielded (and to some degree still yield) only a few fascinating results and many unresolved questions. Nevertheless, the time had come to try to help move the molecular biology of cognition a step forward.
In examining implicit memory in Aplysia, I had built a neurobiolog-ical and molecular approach to elementary mental processes on a foundation laid by Pavlov and the behaviorists. Their methods were
