
3291
.pdf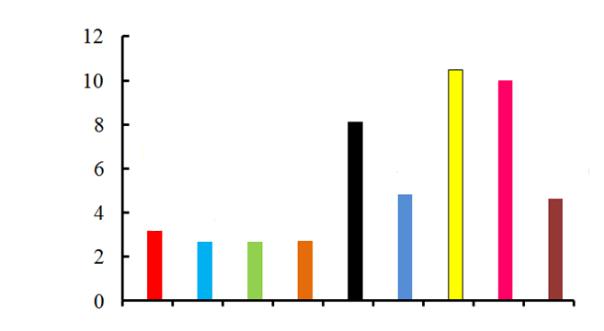
Issue № 3(31), 2016 |
ISSN 2075-0811 |
Among polyelectrolytes the following anionites have been studied:
––strong-base — gel on a sterol divinyl base АV-17-8, porous АРА-2P and poorly crosslinked (2 % divinylbenzene) АV-17-2P;
––strong-base anionite Purolite A100;
––medium-base anionite Wofatit AD-41;
––bifunctional anionite containing ionogenic groups, strongly and weakly dissociating, EDE-10P;
––weak-base anionite АN-31, etc.
At the first stage some physical and chemical properties of anionites that were important for further research were investigated such as a total exchange capacity, number of strongand weak-base functional groups, moisture capacity of a swelled sample in different ion forms.
One of the major indicators of the quality of ionites is the number of functional groups, i.e. a total exchange capacity, i.e. the number of all the functional groups that can participate in an ion exchange, i.e. in a reaction:
R-Cl + (С18Н23SO3)– Na+ = R-(С18Н23SO3) + NaC1;
R-ОН + (С18Н23SO3)– Na+ = R-(С18Н23SO3) + NaОН.
I.e. an organic ion replaces ОНor the chloride-ion in an anionite and this is an ion exchange. Total exchange capacities are in Fig. 1.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EDE-10P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AN-31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Exchange capacity, mg-eq/g |
|
|
|
|
|
|
|
|
|
AD-41 |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Purolite A100 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
AV-17-2P |
|
|
|
Purolite A400 |
|
|
|
|
|
|
|
|
AN-2F |
||||
|
|
AV-29-12P |
|
|
ARA 2PT |
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 1. Total exchange capacities of anionites with different bases
Anionites with up to 97 % low-base groups turned out to have the highest exchange capacity,
which are capable of ion exchange, e.g., salt or chloride [12].
31
Scientific Herald of the Voronezh State University of Architecture and Civil Engineering. Construction and Architecture
3. Kinetic performance. While choosing a suitable ionite we had to bear in mind that sorption should take as little time as possible, i.e. those ionites that have proper kinetic indicators – high constants of absorption rate as shown in [13].
Proper kinetic indicators are vital to the performance of an absorber: the faster a substance is absorbed, the less time it takes for water and a sorbent to come in contact and the better a water purification device will operate. Furthermore even at a high flow rate, i.e. even over a short time that water stays in the ionite layer providing highly effective sorption of a substance.
The parameters that kinetic and dynamic [14, 15] characteristics of the property of an anionexchanger, i.e. an ion shape, granulometric composition, temperature, reaction of a medium, ionic strength of a solution, moisture content, etc. depend on [16]. In order to evaluate them, the laws of kinetics of absorption of nekal by anionites obtained by means of polymerization and polycondensation were investigated. They have a different shape of granules: polymerization ones have a regular spherical shape, polycondensation ones –– an irregular one with curves and bends and sharp angles where there is usually most surface energy, which might affect the sorption capacity of a sample. Anionites were in the form of a hydroxide (ОН-) and salt (Сl-) ions. The granulometric composition of sorbents are represented by fractions with the diameter of the granules 0,25—0,60; 0,60—1,20; 1,20—1,5 mm.
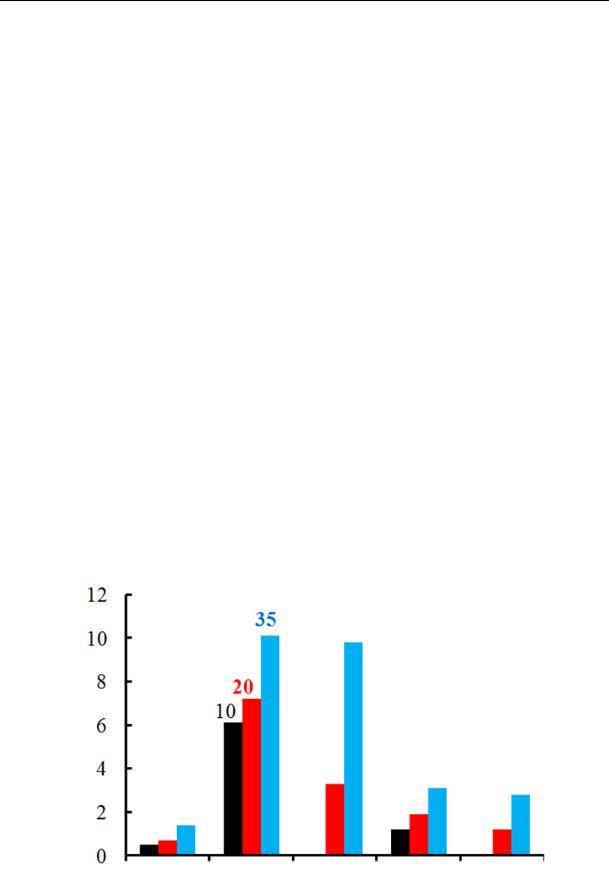
The sorption rate of nekal increases as does the temperature and transition of anionite into salt [17—19]. The dependence of a rate constant on the temperature is identical in all of the investigated anionites as shown in Fig. 2: the sorption rate of nekal increases in all of the samples.
|
|
|
|
AD-41 |
|
|
|
|
|
|
–1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EDE-10P |
|
|
|
|
|
|
|
|
|
|
A100Purolite |
|
|
|||
10·k |
|
2P-17-AV |
|
|
|
|
|
|
||
|
|
|
|
|
|
31-AN |
||||
c , |
|
|
|
|
|
|
|
|
|
|
5– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 2. Rate constants for sorption of nekal by anionites at the temperatures 10, 20 and 35 оС
32
Issue № 3(31), 2016 |
ISSN 2075-0811 |
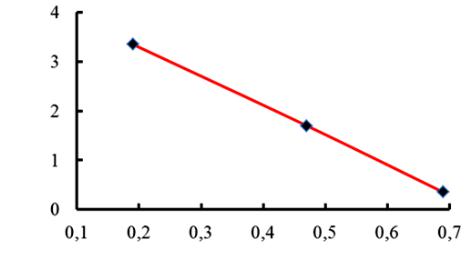
4. Sorption capacity of a sorbent in organic substances depends on the total area of the surface of loading of a filter. The smaller the granules are, the larger the surface area is. In order to estimate the influence of a granulometric composition on the sorption of nekal the rate constants were computed using the experimental data. Fig. 3 represents the dependence of a sorption rate constant on the size of a grain using the example of a weak-base anionite EDE-10P.
k · 10–5, c–1
Radius, mm
Fig. 3. Dependence of a sorption rate constant of nekal with the anionite EDE-10P
in ОН on the size of ionite particles
As the radius of an anionite grain decreases from 0,7 to 0,2 mm, the sorption rate goes up by 6—7 times. The difference is not so significant for a strong-base anionite. This suggests that as a filter is loaded with a sorbent, its large fractions should be put aside as it absorbs nekal poorly as it occupies the space thus compromising the quality of water purification. Despite the fact that grinding of the anionite promotes the growth of the rate constant of nekal, an insignificant decrease in the size of the ionite particles causes an increase in the hydraulic resistance of the filter nozzle. Therefore it makes no sense to keep grinding the sorbent.
Fig. 1 shows the total exchange capacity of anionites, i.e. the number of functional groups that can absorb the ions due to ion exchange. But not all active groups take part in the sorption of large organic molecules. A lot of pores of anionites are simply unavailable to the particles being absorbed due to some steric problems. Therefore the total sorption capacity in such cases must be determined in relation to a certain substance being nekal in this case.
The sorption capacity is shown in Table 1.
33

Scientific Herald of the Voronezh State University of Architecture and Civil Engineering. Construction and Architecture
|
|
|
|
|
|
Table 1 |
|
Sorption capacity of anionites for nekal, Т = 296 К |
|
|
|||
|
|
|
|
|
|
|
Indicator |
|
|
Cl anionite |
|
|
|
|
|
|
|
|
|
|
|
|
Wofatit AD-41 |
Purolite A100 |
|
АV-17-2P |
АV-29-12P |
|
|
|
|
|
|
|
а, mmole/g |
|
1,10 |
0,65 |
|
0,42 |
0,15 |
|
|
|
|
|
|
|
а, mg/g |
|
376,2 |
222,2 |
|
143,6 |
51,3 |
|
|
|
|
|
|
|
MPC, mmole eq/g |
|
8,14 |
4,83 |
|
3,16 |
2,70 |
|
|
|
|
|
|
|
Moisture content, g Н2О/g |
|
1,60 |
1,20 |
|
2,85 |
1,10 |
|
|
|
|
|
|
|
Table 1 suggest that according to the sorption capacity for nekal anionites are ordered as follows:
Wofatit AD-41 > Purolite A100 > АV-17-2P > АV-29-12P.
It is obvious that for anionites on an identical polymer matrix obtained by means of polymerization there is a certain correlation between the sorption capacity and total exchange capacity, i.e. with the number of functional groups in an ionite: the larger MPC is, the more nekal is absorbed.
As the anionites in the experiment were in a salt ion form, we can assume that this result is largely due to ion-exchange component of absorption. Neither exchange or sorption capacity depends on the water content in the swelled ionite.
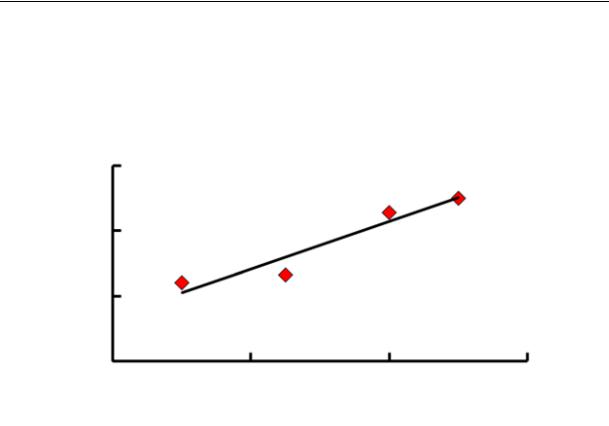
Wofatit AD-41 has a large rate constant compared to the other (Fig. 2) and a high sorption capacity (see Table 1). Fig. 4 shows the dependence of the amount of nekal absorbed from its solutions of different concentration at different temperatures.
a, mmole/g
1,5
1
1,2
0,9
2
0,6
0,3
0
0 |
0,1 |
0,2 |
0,3 |
0,4 |
0,5 |
|
|
Cp, mmole/l |
|
|
|
Fig. 4. Isotherms of absoprtion of nekal by the CI anionite Wofatit AD-41 at the temperature 35 (1) and 23 (2) оС
34

Issue № 3(31), 2016 |
ISSN 2075-0811 |
Based on the position of the curved lines, an increase in the temperature causes a grow in the absorption (а) in the anionite Wofatit AD-41. It is clear that as a nekal solution is heated up from 23 to 35 оС, the sorption capacity of the sorbent in relation to nekal goes up from 376 to 410 mg/l, i.e. almost by 10 %.
Absorption isotherms of nekal involving weak-base anionites have the shape of the Langmuir isotherm. Note that weak-base anionites are more organiс than strong-base ones such as АV- 17-2P and АV-29-12P (see Table 1).
A reaction of a medium of purified water can be different if water is waste. The dependence of the sorption of nekal on рН was found using the example of the anionite CI Wofatit AD-41. Water solutions NaОН and HCI with the рН of 1—12 were prepared for the experiment. Then they were diluted two times in volumetric flasks by a nekal solution with the concentration 1,17 mmole/l. This method of preparing operating solutions provided the equality of the concentrations of nekal in all of the samples. Then identical samples of an air dry anionite were put into each volumetric flask and constantly mixed over 8 hours. After a day the remaining concentration of nekal was identified. The results of absorption are presented in Fig. 5.
a, mmole/g
200
150
100
0 |
2 |
4 |
6 |
8 |
10 |
12 |
14 |
|
|
|
|
pH |
|
|
|
|
|
|
|
|
|
|
|
Fig. 5. Dependence of absorption of nekal with the anionite
Wofatit AD-41on the рН of the solution
It was experimentally shown that a drop in the sorption of nekal can be both in high-alkaline and high-acid media. A more significant drop in sorption is found as pH increases, i.e. in an alkaline medium. This accounts for its capacity to desorb nekal while processing anionites with alkaline solutions.
Sorption can be influenced by the ionic strength of a solution which is determined by the concentration of mineral impurities in a nekal solution. The influence of the ionic strength
35
Scientific Herald of the Voronezh State University of Architecture and Civil Engineering. Construction and Architecture
of the solution was investigated when there was a change in the concentration of NaCI in the range of 0,01—0,1 mole/l. Sorption of nekal from the solution with its content of 0,585 mmole/l (200,0 mg/l) was performed on the anionite CI Wofatit AD-41 at 22 оС. The dependence of absorption on the concentration of the salt is almost linear (Fig. 6).
a, mmole/g
0,6
0,5
0,4
0,3
0 0,02 0,04 0,06 С salt, mole/l
Fig. 6. Dependence of sorption of nekal with the anionite CI
Wofatit AD-41 on the concentration of sodium chloride
Absorption of nekal increases as so does the ionic strength of the solution, which might suggest a sorptive can be effectively removed from water even with a high salt content.
Hence a few studies of kinetic and equilibrium properties of a range of samples identified a type of anionites that can be used to purify water from nekal. They must contain functional tertiary amides. It was found that these anionites should be used as a salt with the pH range from 2 to 9. An increase in the salt content in water does not reduce but increases the capacity of anionites compared to nekal, which is good as that allows waste water with the salt concentration of up to 100 mmole/l and more to be purified. This can be made more effective by heating up water. The anionite Wofatit AD-41 on a sterol matrix was shown to be most optimal of all the samples.
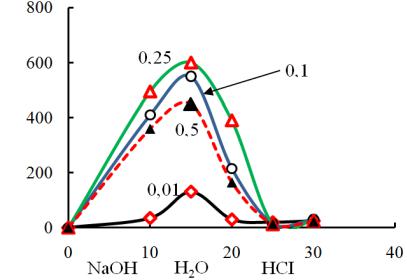
5. Desorption of nekal. Choosing a particular type of anionite is also as was shown in previous experiments due to the fact that unlike other anionites it was found to be capable of desorbing nekal without any solvents contrary to [10, 11]. Different reagents were stested but alkaline solutions were the only to be found to be effective. An optimal concentration of NaOH solution was found according to the maximum principle in Fig. 7.
36
Issue № 3(31), 2016 |
ISSN 2075-0811 |
C, mg/l
V, vol/vol
Fig. 7. Output curved line of the regeneration of anionite Wofatit AD-41 of nekal with the solution of NaOH with a different concentration: С is the concentration of nekal in a regenerate;
V is the volume of solutions and water in loading volumes; numbers of the curved lines are the concentrations of alkali in a solution
It is seen that 0,25 mole/l of the alkali solution displaces more nekal from the anionite than the solution 0,5 mole/l. In this experiment the first 10 volumes are the alkali solution and the second 10 volumes are washing water and the next 10 volumes are the acid solution and then acid washing water (Fig. 7).
According to the identified laws of absorption of nekal, endurance tests of anionite Wofatit AD-41 were carried out at the flow rate of 12 m/h and the height of the layer of 10 сm.
6. Purification from nekal involves a series of operations. Sorption purification water is fed into an absorber filled with an anionite. Purified water is taken out of the absorber with some of it damped into a pipe and the rest collected into purified water from where it is used to prepare alkali and acid solutions and to wash the layer of its reagents.
The anionite is regenerated with the alkali solution. The regenerate containing a desorbed nekal and excessive alkali is fed into an electrodialysis device where the alkali is taken out of the regenerator into a concentration chamber. According to our experimental data, it contains 0,13—0,14 mole/l of OH. The solution is collected into a tank. A solid alkali of 0,1 mole per 1 l is then added. As a result the concentration of the alkali goes up to the necessary 0,25 mole/l. Pure water is not used to prepare an alkali solution.
The regenerated anionite is washed from the alkali. Washing alkali-containing water is also fed into an electrodialyzer. A hydrochloric acid solution is passed through the anionite
37

Scientific Herald of the Voronezh State University of Architecture and Civil Engineering. Construction and Architecture
washed from the alkali for it to be transformed into a salt dissociated ion form. The acid solution does not contain nekal. The first portions of the dripping acid are neutral as OH-anionite interacts with the acid according to the following reaction:
R-OH + HCl = R-Cl + H2О.
This water can be damped into a pipeline and the subsequent acid portions can be collected into a tank and the concentration of the solution is further increased up to 0,5 mole/l. I.e. pure water is not employed during purification. Nekal will be washed from salts and can be used in manifacturing or utilized.
After 15 sorption-desorption cycles water was steadily purified with the nekal content 80— 95 mg/l: 75-92% of 300 volumes of a nekal solution were purified with a loading volume in each filtration cycle [20]. As the flow rate drops to 6 m/h, up to 95—98 % is purified. According to the experimental data, an approximate consumption of reagents and water to purify 1 m3 of water with a high nekal content was identified (Table 2).
Table 2
Consumption of reagents and water for sorption purification of 1 m3 of water from nekal
Reagent |
First regeneration |
Further regenerations |
|
|
|
NаOH, g |
83 |
40 |
|
|
|
НСl, g |
120 |
37 |
|
|
|
Purified water, l |
0 |
34 |
|
|
|
Fresh water, l |
34 |
0 |
|
|
|
Conclusions
The suggested process of sorption purification of water using a weak-base anionite allowing water to be removed at a linear flow rate of 5—6 m/h to the amximum allowable concentration requiring no pure water for the device and ruling out damping of alkali and acid waste water makes it possible for the emitted surface active substances to be utilized or brought back into manufacturing.
In order to specify the conditions of purification of nekal of actual water of water supply wells but not of modelled solutions and more importantly, to prove regeneration and utiliz a- tion of alkali by means of electrodialysis, there should be more endurance tests to see how much an anionite is capable of retaining the sorption capacity and to evaluate its wear and tear resistance. Furthermore the consumption of alkali and acid to purify 1 m3 of water and
38
Issue № 3(31), 2016 |
ISSN 2075-0811 |
their dependence on the concentration of nekal in water, etc., and the influence of other organic impurities on the sorption of nekal need to be identified. It is also recommended that types of other anionites with necessary properties are to be determined.
References
1.Ramad F. Osnovy prikladnoy ekologii [Fundamentals of applied ecology]. Leningrad, Gidrometeoizdat Publ., 1981. p. 543.
2.Levina E. N., Gadanskaya I. D., eds. Vrednye veshchestva v promyshlennosti: organicheskie veshchestva. Novye dannye s 1974 po 1984 gg.: spravochnik [Harmful substances in industry: organic matter. New data from 1974 to 1984: a Handbook]. Leningrad, Khimiya Publ., 1985. 464 p.
3.Kholmberg K., Yensson B., Kronberg B., Lindman B. Poverkhnostno-aktivnye veshchestva i polimery v vodnykh rastvorakh [Surfactants and polymers in aqueous solutions]. Moscow, BINOM-Laboratoriya znaniy Publ., 2007. 528 p.
4.SanPiN 2.1.4.1074-01. Sanitarnye pravila i normy kachestva pit'evoy vody. [Sanitary norms and rules 2.1.4.1074-01. Sanitary rules and norms for drinking water quality]. Moscow, Federal'nyy tsentr Gossanepidnadzora Publ., 2002. 103 p.
5.Kozlov A. T., ed. Doklad o sostoyanii okruzhayushchey sredy i prirodookhrannoy deyatel'nosti gorodskogo okruga gorod Voronezh v 2006 godu [A report on the state of the environment and conservation of the urban district of Voronezh in 2006]. Voronezh, 2007. 64 p.
6.Zinov'ev L. S. Informatsionnyy byulleten' instituta imeni F. Erismana [Newsletter of the Institute named after
F.Erisman], 1956, vol. 3, pp. 3—6.
7.Ivanov V. A. [Impact on groundwater liquid discharge plant SK]. Trudy Voronezhskogo otdeleniya VKhO im.
D.I. Mendeleeva [Proceedings of the Voronezh branch of VKHO im. D. I. Mendeleev]. Voronezh, 1959, pp. 175—179.
8.Kalugina N. V. Monitoring podzemnykh vod [Groundwater monitoring]. Vestnik Voronezh. gos. un-ta. Geologiya, 2003, no. 2, pp. 237—239.
9.«Myl'noe ozero» pod Voronezhem — prichina defitsita vody v kranakh ["Soap lake" near Voronezh — the reason for the shortage of water in the taps]. Available at: http://vestivrn.ru/novosti/myilnoe-ozero-pod- voronezhem-prichina-defitsita-vodyi-v-kranah_2009-8-18_18-30
10.Kurolap N. S. Izuchenie zakonomernostey sorbtsii poverkhnostno-aktivnykh veshchestv anionnogo tipa ionoobmennymi smolami. Avtoref. diss. kand. khim. nauk [Study of sorption regularities of surface-active substances of anionic-type ion exchange resins. Abstract cand. chem. scie. diss.]. Voronezh, 1970. 24 p.
11.Valashek Yu., Pogl B., Zedek S. [Wastewater from the production divinylacetylene rubber]. Trudy VODGEO "Ochistka promyshlennyh stochnyh vod" [Proc. of VODGEO "Industrial wastewater treatment"]. Moscow, 1960, pp. 72—82.
12.Kurenkova O. V. Sorbcionnoe izvlechenie anionnogo PAV dibutilnaftalinsul'fonata natrija iz podzemnyh i stochnyh vod. Avtoref. diss. kand. khim. nauk [Sorption extraction of anionic surfactants dibutyldithiocarbamate of sodium from groundwater and wastewater. Abstract cand. chem. scie. diss.]. Moscow, 2011. 18 p.
39

Scientific Herald of the Voronezh State University of Architecture and Civil Engineering. Construction and Architecture
13.Slavinskaja G. V., Kurenkova O. V. Vybor anionita dlja udalenija iz vody nekalja po kineticheskim harakteristikam ego sorbcii [The choice of the anion exchanger for removal of water Nacala kinetic characteristics of sorption]. Nauchnyy vestnik Voronezhskogo GASU. Stroitel'stvo i arkhitektura, 2012, no. 3 (27), pp. 56—65.
14.Kotova D. L., Krysanova T. A., Davydova E. G. Vliyanie temperatury na dinamicheskie kharakteristiki sorbtsii prolina i gidroprolina na N-sul'fokationoobmennike KU-2-8 [The effect of temperature on the dynamic characteristics of adsorption of Proline and hydroproline the H-sulfocationite KU-2-8]. Sorbtsionnye i khromatograficheskie protsessy, 2009, vol. 9, no. 2, pp. 241—246.
15.Slavinskaya G. V., Zeleneva L. A., Kuznetsova N. S. [Analysis of the unit operation of ion exchange desalting of natural waters]. Trudy «Teoriya i praktika sorbtsionnykh protsessov» [Proc. of the Theory and practice of sorption processes], Voronezh, Izd-vo Voronezh. gos. un-ta, 1983, vol. 16, pp. 101—105.
16.Koganovskiy A. M., Klimenko N. A., Levchenko T. M. Adsorbtsiya organicheskikh veshchestv iz vody [Adsorption of organic substances from water]. Leningrad, Khimiya Publ., 1990. 256 p.
17.Slavinskaya G. V., Kurenkova O. V. Kinetika sorbtsii anionnogo PAV anionita na epikhlorgidrinovoy matritse [The kinetics of sorption of anionic surfactants on anion-exchange resins epichlorhydrin matrix].
Sorbtsionnye i khromatograficheskie protsessy, 2011, vol. 11, iss. 2, pp. 204—208
18.Kurenkova O. V., Slavinskaya G. V. Issledovanie mekhanizma kinetiki sorbtsii dibutilnaftalinsul'fonata natriya polielektrolitami [Investigation of the mechanism of sorption kinetics dibutyldithiocarbamate sodium polyelectrolytes]. Sorbtsionnye i khromatograficheskie protsessy, 2009, vol. 9, iss. 6, pp. 844—852.
19.Slavinskaya G. V., Kovaleva O. V. Issledovanie zakonomernostey kinetiki sorbtsii dibutilnaftalinsul'fonata natriya polielektrolitami [The study of regularities of kinetics sorption dibutyldithiocarbamate sodium polyelectrolytes]. Sorbtsionnye i khromatograficheskie protsessy, 2009, vol. 9, iss. 4, pp. 521—528.
20.Slavinskaya G. V., Kurenkova O. V. Issledovanie dinamiki sorbtsii dibutilnaftalinsul'fonata natriya (nekalya) polielektrolitami [Study of the dynamics of sorption dibutyldithiocarbamate sodium (nekala) polyelectrolytes].
Sorbtsionnye i khromatograficheskie protsessy, 2014, vol. 14, iss. 5, pp. 703—711.
21. Seredin P. V. Spinodal'nyy raspad v epitaksial'nykh tverdykh rastvorakh geterostruktur ALXG1XAS/GAAS(100) i GAXIN1-XP/GAAS(100). Izvestiya Samarskogo nauchnogo tsentra Rossiyskoy akademii nauk, 2009, vol. 11, no. 3––1, pp. 46––52.
40
