
3202
.pdfIssue № 3 (35), 2017 |
ISSN 2542-0526 |
1 g/l) but also organic ones both qualitatively and quantitavely: chromaticity of 20 degrees of the chromecobalt scale and the permanganate index of 5 mg О2/l; in concrete works water is used to prepare concrete mixes, watering concrete for solidification, washing fillers, etc. For all of these not any water is appropriate but only the one that is in accordance with the technical requirements specified in the State Standards (GOST) 23732-79. This is drinking water as well as that from rivers and lakes that is good for use in solving a concrete mix, etc.
Water oxidability cannot be over 15 mg О2/l. Water pH value should range from 4 to 12.5. A neutral medium is characterized by рН = 7. At рН < 7 a medium is acid, at рН > 7 it is alkaline. Therefore water with a subacid or subalkaline reaction can be used for solving. Water containing humic or other organic acids can affect concrete solidification. Water should be colourless or yellowish with the chromaticity of no more than 70° according to the State Standards (GOST)-3351. If there are requirements for technical aesthetics of concrete, the chromaticity of water should not be over 30°.
Organic substances particularly sugars and phenols are known to slow down regular hydratation of cement and thus reduce the concrete strength. Therefore there should be no more than 10 mg/l of each in water used for solving. Water containing foam-forming surfactants is good if the foam strength is no longer than 2 min [5].
Hence while preparing drinking water as well as technological one, it is necessary that organic substances are removed including coloured humic and fulvic acids as well as surfactants if there are more of these than specified in the corresponding standards. In practice these substances are easily removed through a layer of synthetic anion-exchangers [6, 14, 17, 29, 32, 34, 37].
However, repetitive operating cycles of purification need a restored sorption capacity of loading filters so solutions of alkali, salts and their mixes are necessary [6, 35]. Whether a technology is environment-friendly depends on how often regeneration takes places and how much wastewater is formed in the process. In order to reduce the number of regenerations, filtering cycles have to be long [6, 17, 23]. Therefore it is necessary to identify the mode to allow to make as much use of the sorption capacity of a filtering layer of an ion-exchanger as possible.
A medium reaction can be designed using all the external parameters in original or processed water to intensity the sorption of impurities. If рН is optimal, the quality of purified water is improved provided that a cycle is longer and the amount of released acid and alkaline wastewater is lower. The content of fulvic acids is known to be invariably 10…30 times high-
51

Russian Journal of Building Construction and Architecture
er than that of humic acids and other substances [6, 18, 24]. Therefore the studies were performed for fulvic acids as well as anion nekal surfactant.
Identifying how much рН influences the sorption of anion surfactants is of both theoretical and practical interest: by varying the parameter, a surfactant can be modified to enhance sorption. This particular series of experiment was aimed at determine the dependence of sorption of fulvic acids of natural waters and nekal on рН anionites with functional groups of different ionization degrees.
1. Extracting and purifying water fulvic acids
Developing the technology of removing fulvic acids is impossible without quantitative estimation of changes of their concentration duting water purification employing a variety of methods. These acids are not industrially produced and were thus extracted from some water reservoirs [20].
It was noted [36] that when natural water is bleached by liming, it loses some coloured humic acids. This is a foundation of the method of extracting humic acids developed by Novocherkassk Hydrochemical Institute where fulvic and humic acids are mutually precipitated resulting in a precipitation that is mineral and insoluble in water.
In order to obtain it, 60 g of Na2CO3 was solved in one portion of the processed river water (10 l) and 100 g of CaCI2 in another one. This ratio of the substances was selected so that following bleaching the liquid had the рН~7. If a medium reaction is acid, humic acids will be precipitated, if it is alkaline, they will solve with an organic salt, i.e. natrium humate.
The resulting
Na2CO3 + CaCI2 = 2NaCI + СаСО3 calcium carbonate was solved in HCI:
СaCO3 + 2НCI = CaCI2 + Н2О + СО2.
After рН reaches ~1, humic acids were released (precipitation time is 12 days). Following their extraction by means of centrifugation, fulvic acids were left in the solution that are solved well in an acid medium as well as NaCI, CaCI2 and HCI. The acid and salt were removed using the method of ion exchange using tight cations and anionites. Ion-exchangers were regenerated between the cycles using solutions of reagents with the concentration 1 mole/l: НCI (cationite) and NaOH (anionite).
Fulvic acids are known to be purified using different gels [27, 30, 33]. For desalination aliquots of the solution were repeatedly released through a series of cationites and anionites until the mineral substances were completely removed. Finally, the solution was filtered through mixed
52

Issue № 3 (35), 2017 |
ISSN 2542-0526 |
loading that consisted of an H-cationite and OH-anionite. Purification of the fulvic acid solution from mineral impurities was determined using the specific electrical resistance ( , Оhm/сm). The electrical conducitivity of the system is largely determined by electrolytes: hydrochloric acid, chlorides of metals and fulvic acids proper. The value of the original liquid ( 1) was measured and then that of the resulting one as it passed through an ionite column ( 2). The equality ( 1 = 2) and more importantly, no changes in this index suggest that the specific electrical resistance is only due to fulvic acids whose concentration was stable in the solution despite a contact with the ion exchangers. The latter commonly absorb fulvic and humic acids, which is reported in some papers to be the factor of “aging” of desalinating cationites and anionites [4, 8, 21, 25].
In order to avoid that, ionites are preliminarily “saturated” with fulvic acids and there are thus no changes in their content during filtration through ionites.
Following evaporation of the solution in a vacuum, a solid fulvic acid product was obtained that did not contain any mineral impurities. It was necessary that desalination was thorough and elaborate since the concentration of fulvic acids was determined using spectography and the gradient graphs were designed using excessive weights with the accuracy of 0.0002 g.
2. Identification of the extracted fulvic acids
It appeared possible to identify the resulting product as that of fulvic acid and thus the physical and chemical properties of this substance had to be investigated and compared with available data.
The latter are generally concerned with fulvic acids extracted from soils. Fulvic acids extracted from water are not sufficiently researched.
Table 1 shows the element composition of fulvic acids that we extracted from soil at different times as well as those described in literature.
|
|
|
|
|
|
Таble 1 |
|
Element composition of the extracted fulvic acids |
|
|
|||
|
|
|
|
|
H, % |
|
Source |
|
С, % |
N, % |
O, % |
||
|
|
|
|
|
5.3 |
|
№ 1 (the Neva) |
|
48.8 |
2.4 |
|
43.5 |
|
|
|
|
|
|
6.0 |
|
№ 2 (the Neva) |
|
47.7 |
2.1 |
|
43.6 |
|
|
|
|
|
|
5.1 |
|
№ 3 (the Neva) |
|
45.3 |
1.9 |
|
47.7 |
|
|
|
|
|
|
3.7 |
|
The Usmanka (Voronezh region) |
|
42.3 |
–– |
|
–– |
|
|
|
|
|
|
5.3 |
|
The Ülemiste Lake (Таllinn) |
|
51.6 |
–– |
|
–– |
|
|
|
|
|
|
5.7—6.9 |
|
[26, 30] |
|
45—53 |
2.3—4.1 |
|
42—49 |
|
|
|
|
|
|
|
|
53

Russian Journal of Building Construction and Architecture
Comparing the data we argue that our product of fulvic acid has the element composition that is in the same range as specified in literature.
According to the data it provides, fulvic acids consist of a condensed aromatic nuclear and side chains containing alcohol and phenol hydroxyls, carboxyl and methoxyl groups, CH –– aromatic, СН2-, СН3 — aliphatic as well as free radicals and aminogroups. I.e. fulvic acids have acid groups of different degrees – with different dissociation constants (pKa) [10, 31, 38].
The experiments were conducted to identify the number of functional groups of different types and values of their pKa.
In order to identify the number of carboxyle, i.e. СООН-groups, we used the known calciumacetate method. The total number of acid groups was determined using the method [10]. Fulvic acid was found to range inconsiderably: from 7.10 to 8.00 mg-equiv/g of the substance. These data came in handy for choosing weights of fulvic acids and concentrations of titrant when identifying the type of acid groups.
3. Identifying dissociation constants of fulvic acids
Data on the power of acid groups are necessary to evaluate processes that might take place with fulvic acids as рН of processed water changes and to understand the behavior of anionites for different values of рН, i.e. to identify the dependence of sorption capacity of anionites of different types in relation to surfactants.
The most convenient and reliable method of identifying the type of functional groups and determining their constants of ionization and exchange capacity is potentiometry [7, 19, 26, 28]. Since humiс acids are polyelectrolytes with weak acid properties [26], their potentionmetry was performed by means of one or more weights. For studying acid propertites of humic acids for interpreting potentionmetry curves, theoretical approaches are employed that are developed for solutions of polyelectrolytes such as of carboxyle polyelectrolytes [12, 13, 15]. In potentiometry curves with active groups of different types with enough differences in ionization constants there are frequently a few oscillations as in this case as well. For quantitative calculations in potentiometry the Henderson-Hasselbalch equation is used [4]:
pKi pH n lg .
The parameter n is associated with electrostatic interaction of functional groups. The sign «+» belongs to acids and «–» to foundations, is an ionization degree of an acid group. The value of рН of an external solution is a linear function [15]:
lg .
54

Issue № 3 (35), 2017 |
ISSN 2542-0526 |
Using the line cut along the рН axis of the straight line, the apparent ionization constant was found. At = 0.5 (regardless of the value n) рКа = рН.
3.1. Potentiometry of fulvic acids
A titrant solution is 0.01 mole/l of КОН. рН was measured using a standard potentiometry tool рН-340. A glass electrode ESL-41G-04 was used for measurements and a flow chlorum chlorsilver electrode for comparison.
In order to protect a reaction cell from СО2 of the air, argon purified by absorbents was blown through the solution. The device was adjusted and the electrodes were evaluated by means of potentiometry of acetic acid (рКа = 4.74). The value of рКа that was found for this acid was (4.74±0.03). Тhis degree of accuracy of determining the ionization constant is considered high [1].
If one weight of fulvic acid undergoes potentiometry directly (Fig. 1а) in three parallel experimetns quite identical values of the original value of рКа were obtained: 4.45; 4.45 and 4.35 and the number of functional groups that equals 4.48; 4.53 and 4.53 mg-equiv/g. All the data suggest that using the method of one weight of fulvic acid only one type of functional groups is determined and that would most likely be carboxyle groups (СООН) that were found to be ionized in fulvic than acetic acids.
V, ml |
|
V, ml |
|
|
|
Fig. 1. Curves of potentiometry of fulvic acids using the method of one weight (а), several weights (b): 1 — differential curves, 2 — integral curves
However, comparing the identified common group of capacities of connection of fulvic acids, i.e. the number of functional groups (7.10—8.00 mg-equiv/g) and the obtained potentiometry data suggest that using direct potentiometry of one weight of fulvic acid only
55

Russian Journal of Building Construction and Architecture
55—65 % of the most ionized functional groups of the product can be determined. These can be СООН-groups of aromatic or aliphatic amino acids that are part of peripheral chains of fulvic acids. The ratio that was found [19, 20, 39, 40] of strong and weak acid groups in fulvic acid is in agreement with the data presented in the papers [10, 13, 28].
During potentiometry using КOH solutions of weak acid groups due to hydrolysis of the resulting salt of the weak acid and strong foundation, balance is achieved slowly. Therefore they cannot be determined using potentiometry of one weight. Thus the number of types of acid groups and their ionization constants was determined using potentiometry of several weights.
For that in dry flasks of 50 ml 10 ml of the fulvic acid solution was put and 0.01 mole/l of the КОН solution increasing the doze by 0.1 ml in each subsequent test. The flasks were then tightly shut. Two glass tubes were inserted into each: argon was supplied in the one that was submerged down to the liquid layer and it was released into the atmosphere through the other (short) one. After the blowing, the tube taps were shut. The samples isolated from СО2 were kept for a week at (20 1) С. During potentiometry the solution and the dozed КОН solution were mixed using a flow of argon protecting the system from contacting СО2 of the air.
According to the results of the experiments, during a long contact of fulvic acids with the alkali there is ionization of not only one but three types of functional groups (Fig. 1b) with рК 4.3; 8.0 and 9.5. Their number is 4.19; 3.35 and 1.68 mg-equiv/g respectively, which is 9.12 mg-equiv/g of fulvic acid in total.
Therefore using potentiometry three types of acid groups of different power are found in fulvic acids extracted from the Neva water, which is identical to what was described in literature [3, 10]. The weakest groups (рКа > 9) are ionized at рН > 11, i.e. in an alkaline medium. That might be phenol hydroxyls that enter a neutralization reaction if a medium is considerably alkaline but they are not determined using the calcium-nitrate method [10]. This is likely why there is a difference between the number of acid groups of fulvic acid found using the method [10] and potentiometry.
Besides identifying the element composition and type of functional groups of fulvic acids UVand IR-absorption spectra were found that proved there are both carboxyle and phenol groups in the product. The composition of amino acids that are included in the peripheral chains of the fulvic acid molecules was also determined [20].
The properties of the obtained fulvic acids were so elaborately and thoroughly researched so that there are no doubts as to whether the product genuinely corresponds with fulvic acids.
56

Issue № 3 (35), 2017 |
ISSN 2542-0526 |
4. Preparation and characteristics of nekal
Fulvic acids belong to natural anionite active surfactants. But synthetic surfactants occur in nature as well. E.g., nekal that has been in use in industry to obtain rubber for decades. Different types of enterprises have contributed to water pollution as well as household wastes that contain various synthetic washing substances. The total concentration of surfactants in drinking water was no more than 0.5 mg/l [5].
An anionic surfactant dibutylnaphthalenenatriumsulfonate (nekal) was employed for the research: a mix of anionic surfactants, i.e. isometric mono, diand tributylnaphthalenenatriumsulfonate containing Na2SO4 and other impurities. The structural formula of nekal (C18H23SO3Na) is in Fig. 2.
Fig. 2. Structural formula of nekal
The fact that there is a heavy organic radical and a strong electrolyte (SO3Na ion) makes it a surfactant, hence the emulgate, foam-forming and washing properties. A salt of a strong foundation and polyatomic organic acid with an unknown ionization constant dissociates with a natrium cation breaking away. I.e. this substance (electrolyte) whose anion is a singly charged particle that is fairly large, i.e. 0.34 nm [9]. The calculation was performed using the method [21].
As nekal is synthesized, some of sulphuric acid remains in the solution and during neutralization using caustic soda it forms sulfuric natrium that reaches 10 % in stock nekal. Additionally, there is some sodium chloride in the solution [20].
After double recrystallization of the product that was conducted in order to remove unnecessary impurities we determined whether there were any salts of strong acids (suphuric and hydrochloric) in nekal using the method of conductometric potentiometry of 0.01 mole/l with a solution of AgNO3. Note that electric conductivity of the nekal solution is due to its dissociation into anions of dibutylnaphthalenenatriumsulfonate acid and natrium cations as well as salts if there are any in the solution. If there are sulphate and chloride-ions, an increase in the amount of the solution of AgNO3 should reduce the electrical conductivity of the system as chlorides and sulphates of silver are insoluble and precipitate. If this takes
57

Russian Journal of Building Construction and Architecture
place, the electrical conductivity ( , reciprocal ohm/m) of the solution should go down as the anions will be released from the solution. But in our study was on the rise (Fig. 3), i.e. the added ions of silver did not precipitate and along with the nitrate-ions contributed to an increase in the electrical conductivity of the system, which proves that there are no anions of sulphuric and hydrochloric acids in the test solution.
, Reciprocal ohm/m
|
V, ml AgNO3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Fig. 3. Curves of conductometric potentiometry of the |
|
, nm |
||||
nekal solutions with the concentration 1.17 (1) |
|
|
|
|||
Fig. 4. Nekal chromatography |
||||||
|
and 2.92 (2) mmole/l |
|||||
|
|
|
|
|||
Besides mineral impurities, there can also be other organic substances in the nekal solution including different derivatives of naphthalenesulfonic acid as well as naphthalene or butyl alcohol and other impurities that might be in the original components during the synthesis of nekal.
In order to determine whether there are some or no molecules of other organic substances or monomers in the fulvic acid product, the method of highly effective liquid chromatography was used. A liquid microtube chromotographer “Milichrome—6” with a multi-wave scanning spectrography detector with the wavelength range 190—360 nm: in the chromatogram (Fig. 4) there is one peak at the wavelength = 254 nm for a small time of keeping, which is typical of ionic compounds, which is nekal.
The certainly as to the cleanness of the nekal (no organic salts and other unnecessary organic components) enabled us to prepare its solutions with precise concentrations using weights of the substance taken on analytical weights with the accuracy of 0.0002 g.
58
Issue № 3 (35), 2017 |
ISSN 2542-0526 |
5. Determining the effect of рН on the sorption of fulvic acids
It was previously established [20] that fulvic acids are absorbed by anionites as a result of physical absorption and ion exchange. The latter is only possible if both the carboxyl groups of fulvic acids and functional groups of the anionite are ionized. For different values of рН not only the state of fulvic acids themselves but also anion exchangers are replaced.
It was mentioned above that there are different original acid groups in fulvic acids as well as carboxyl ones that are more ionized that those in the acetic acids.
Depending on the рН of fulvic acids in the solution there can be protonated molecules (NH2-R-CОOН), zwitter-ions (+NH3-R-CОO-), cations (+NH3-R-CОOH) or anions (NH2-R-CОO-).
Dissociation of functional groups of anionites also depends on the reaction of a medium. In an acid medium (рН=1…2) ionites are in the salt (e.g., chloride) form. This means that regardless of the type of an anion-exchangers all of its groups will dissociate. However, in this range рН molecules are protonated, i.e. the carboxyl groups do not split a hydrogen ion (+NH3-R-CОOH), which makes their sorption impossible due to ion exchange of the ionites of all types and therefore in an acid medium it takes place only thanks to physical absorption.
In a neutral medium when most of fulvic acid dissociates (NH2-R-CОO-), sorption depends on the type of an anionite. Ion exchange is possible in high-foundation anionites as the latter are ionized in the ОН-form, however in this case low-foundation anionites do not take part in the ion exchange reaction as in their main form they cannot split the ОН-groups. But physical absorption of fulvic acids in a neutral edium can take place in anionites of both types with varying degrees of effectiveness.
In an alkaline medium low-foundation anions do not dissociate while all the molecules of fulvic acids transform into anions (NH2-R-CОO-), but ion exchange is impossible, it is only physical sorption that can actually occur. In this range of рН high-foundation anionites being in the ionized state in the ОН-form actively absorb fulvic acids both via ion exchange as well as physical absorption.
The cause of the difference in the sorption of large organic molecules for different counterions can also generally be a change in swelling (V, g Н2О/g of the weighed portion) of anionites in the salt and hydroxyl forms: as it goes up, so does sorption as there are extra opportunities for the molecules of a sorptive substance to be transported at the sorbent phase. But it only happens if an anionite is dissociated.
Therefore the number of fulvic acids that were absorbed based on the ion-exchange mechanism is determined by a combination of opposing factors that are created by a medium reac-
59
Russian Journal of Building Construction and Architecture
tion: an ionized anionite and a non-ionized fulvic acid do not take part in ion exchange and neither does a non-ionized anionite with an ionized fulvic acid. I.e. due to ion exchange sorption is a factor that enhances the sorption capacity of anionites and thus increases the efficiency of water purification.
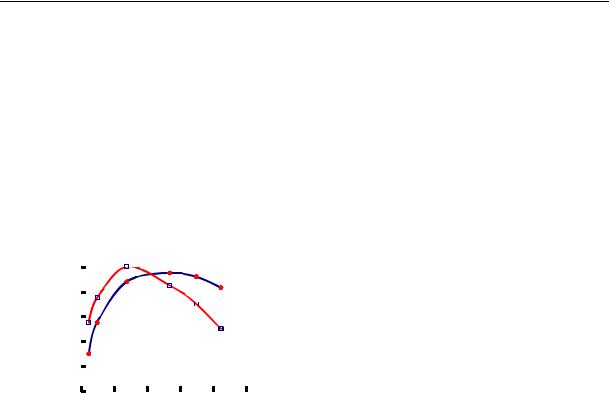
The experiments were conducted in static conditions. Solutions of fulvic acids with the concentration from 27 to 83 mg/l were used. A typical dependence of sorption on рН is shown using the example of anionites: a low-foundation (phenol) IА-3 and high-foundation (styrene) АV-17-8P (Fig. 5) ones.
1 |
|
|
q |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||||
0,8 |
|
|
|
|
|
|
|
|
|
|
|
1 |
|
Fig. 5. Dependence of the effectiveness of sorption |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
0,6 |
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
of fulvic acids by anionites АV-17-8P (1) |
0,4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
and IА-3 (2) on рН: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
0,2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
q — is a sorption proportion of its maximum value |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
рН |
for a particular anionite |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
0 |
2 |
4 |
6 |
8 |
10 |
|
|||||||||
It was found to have its maximum: for the low-foundation anionite at рН 2…5, for the highfoundation one at 3…7. That is essential to know in practice. Based on that, there are recommendations as to where sorbents of organic substances should be placed in a technological chain of natural water purification: a low-foundation anionite should follow H-cation filter and a high-foundation one is suitable after ОН-filters of the first stage of desalination at рН ~ 7 as functional groups of anionites of this type are ionized in a wide range of рН.
Sorption of a substance occurs in time. It depends on the kinetic characteristics of the process. In order to estimate the influence of рН on the kinetics of sorption of fulvic acids, velocity constants (k, c-1) (D) of fulvic acids and diffusion coefficients into an anionite grain (Fig. 6b, 6c) were calculated in another series of experiments (Fig. 6a).
According to the position and shape of the curves (Fig. 6а), the maximum sorption of fulvic acids on the selected anionites as well as in the above experiment (Fig. 5) is seen at рН of about (3 1), i.e. in a weakly acid medium. Ionization constants of functional groups change in a range of anionites: IА-1 < АNT-511 < АV-17-2P [2].
It means (Fig. 6а) that the less ionized functional groups of an anionite are, the less distinctive a drop in sorption is as рН changes in a neutral and alkaline area of рН. This is due to a decrease in ionization of functional groups resulting in a drop in the ion-exchange component, which affects the total sorption of fulvic acids.
60
