
книги студ / Color Atlas of Pharmacology
.pdf
|
|
|
|
Antithrombotics |
151 |
Arachidonic |
Thrombin |
|
|
|
|
acid |
Hirudin |
Serotonin |
PAF |
|
|
|
Argatroban |
|
|
|
|
Thromb- |
|
|
Abciximab |
||
oxane A2 |
|
|
Tirofiban |
|
|
ASA |
|
|
|
Eptifibatide |
|
GPIIB/IIIA |
|
|
ATP |
GPIIB/IIIA |
|
[without |
|
|
[Affinity for |
||
affinity for |
|
|
|
fibrinogen |
|
fibrinogen] |
|
Phospho- |
|
high] |
|
|
|
|
|
|
|
|
|
diesterase |
Adenylate- |
|
|
|
|
|
cyclase |
|
|
Dipyridamole |
ADP |
|
|
|
|
|
|
|
|
||
|
Ticlopidine, Clopidogrel |
|
|
|
|
Inactive |
|
|
|
Active |
|
A. Inhibitors of platelet aggregation
Platelet with |
Platelet |
acetylated and |
|
blocked |
|
cyclooxygenase |
|
Low dose of |
|
acetyl- |
|
salicylic acid |
|
COOH |
|
O |
CCH3 |
|
O |
B. Presystemic inactivation of platelet cyclooxygenase by acetylsalicylic acid
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

152 Plasma Volume Expanders
Plasma Volume Expanders
Major blood loss entails the danger of life-threatening circulatory failure, i.e., hypovolemic shock. The immediate threat results not so much from the loss of erythrocytes, i.e., oxygen carriers, as from the reduction in volume of circulating blood.
To eliminate the threat of shock, replenishment of the circulation is essential. With moderate loss of blood, administration of a plasma volume expander may be sufficient. Blood plasma consists basically of water, electrolytes, and plasma proteins. However, a plasma substitute need not contain plasma proteins. These can be suitably re- placed with macromolecules (“colloids”) that, like plasma proteins, (1) do not readily leave the circulation and are poorly filtrable in the renal glomerulus; and (2) bind water along with its solutes due to their colloid osmotic properties. In this manner, they will maintain circulatory filling pressure for many hours. On the other hand, volume substitution is only transiently needed and therefore complete elimination of these colloids from the body is clearly desirable.
Compared with whole blood or plasma, plasma substitutes offer several advantages: they can be produced more easily and at lower cost, have a longer shelf life, and are free of pathogens such as hepatitis B or C or AIDS viruses.
Three colloids are currently employed as plasma volume expanders— the two polysaccharides, dextran and hydroxyethyl starch, as well as the polypeptide, gelatin.
Dextran is a glucose polymer formed by bacteria and linked by a 1!6 instead of the typical 1!4 bond. Commercial solutions contain dextran of a mean molecular weight of 70 kDa (dextran 70) or 40 kDa (lower-molecular- weight dextran, dextran 40). The chain length of single molecules, however, varies widely. Smaller dextran molecules can be filtered at the glomerulus and slowly excreted in urine; the larger ones are eventually taken up and de-
graded by cells of the reticuloendothelial system. Apart from restoring blood volume, dextran solutions are used for hemodilution in the management of blood flow disorders.
As for microcirculatory improvement, it is occasionally emphasized that low-molecular-weight dextran, unlike dextran 70, may directly reduce the aggregability of erythrocytes by altering their surface properties. With prolonged use, larger molecules will accumulate due to the more rapid renal excretion of the smaller ones. Consequently, the molecular weight of dextran circulating in blood will tend towards a higher mean molecular weight with the passage of time.
The most important adverse effect results from the antigenicity of dextrans, which may lead to an anaphylac- tic reaction.
Hydroxyethyl starch (hetastarch) is produced from starch. By virtue of its hydroxyethyl groups, it is metabolized more slowly and retained significantly longer in blood than would be the case with infused starch. Hydroxyethyl starch resembles dextrans in terms of its pharmacological properties and therapeutic applications.
Gelatin colloids consist of crosslinked peptide chains obtained from collagen. They are employed for blood replacement, but not for hemodilution, in circulatory disturbances.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Plasma Volume Expanders 153
|
Circulation |
|
||
|
|
Blood loss |
danger of shock |
|
Gelatin colloids |
|
|
||
= cross-linked peptide chains |
Plasma |
|||
MW 35, 000 |
||||
|
|
|||
|
|
Plasma- |
||
|
|
proteins |
||
Peptides MW ~ 15, 000 |
|
|
||
Gelatin |
MW ~ 100, 000 |
|
|
|
Collagen MW ~ 300, 000 |
|
|
||
|
Dextran |
|
|
|
|
Plasma- |
|
Erythrocytes |
|
|
substitute |
|
||
|
with colloids |
|
||
|
|
Hydroxyethyl starch |
||
|
|
MW 450, 000 |
||
Sucrose |
Fructose |
|
|
|
|
|
|
||
|
Bacterium |
Hydroxy- |
|
|
|
Leuconostoc |
|
||
|
mesenteroides |
ethylation |
|
|
|
|
Starch |
|
|
A. Plasma substitutes |
|
|
||
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

154 liDrugsKT used in Hyperlipoproteinemias
Lipid-Lowering Agents
Triglycerides and cholesterol are essential constituents of the organism. Among other things, triglycerides represent a form of energy store and cholesterol is a basic building block of biological membranes. Both lipids are water insoluble and require appropriate trans-
port vehicles in the aqueous media of lymph and blood. To this end, small amounts of lipid are coated with a layer of phospholipids, embedded in which are additional proteins—the apolipoproteins (A). According to the amount and the composition of stored lipids, as well as the type of apolipoprotein, one distinguishes 4 transport forms:
|
Origin |
Density |
Mean sojourn |
Diameter |
|
|
|
in blood |
(nm) |
|
|
|
plasma (h) |
|
|
|
|
|
|
Chylomicron |
Gut epithelium |
<1.006 |
0.2 |
500 |
|
|
|
|
|
VLDL particle |
liver |
0.95 –1.006 |
3 |
100–200 |
|
|
|
|
|
LDL particle |
(blood) |
1.006–1.063 |
50 |
25 |
|
|
|
|
|
HDL particle |
liver |
1.063–1.210 |
– |
5–10 |
|
|
|
|
|
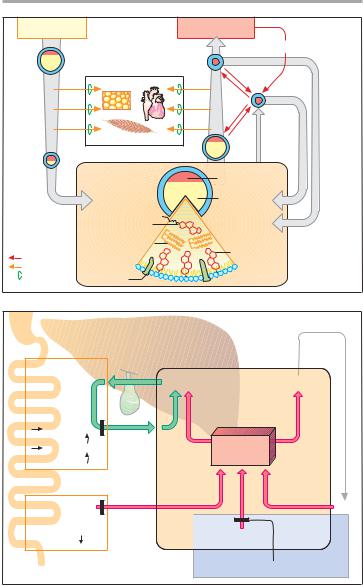
Lipoprotein metabolism. Enterocytes release absorbed lipids in the form of triglyceride-rich chylomicrons. Bypassing the liver, these enter the circulation mainly via the lymph and are hydrolyzed by extrahepatic endothelial lipoprotein lipases to liberate fatty acids. The remnant particles move on into liver cells and supply these with cholesterol of dietary origin.
The liver meets the larger part (60%) of its requirement for cholesterol by de novo synthesis from acetylcoen- zyme-A. Synthesis rate is regulated at the step leading from hydroxymethylglutaryl CoA (HMG CoA) to mevalonic acid (p. 157A), with HMG CoA reductase as the rate-limiting enzyme.
The liver requires cholesterol for synthesizing VLDL particles and bile acids. Triglyceride-rich VLDL particles are released into the blood and, like the chylomicrons, supply other tissues with fatty acids. Left behind are LDL particles that either return into the liver or supply extrahepatic tissues with cholesterol.
LDL particles carry apolipoprotein B 100, by which they are bound to receptors that mediate uptake of LDL into the
cells, including the hepatocytes (recep- tor-mediated endocytosis, p. 27).
HDL particles are able to transfer cholesterol from tissue cells to LDL particles. In this way, cholesterol is transported from tissues to the liver.
Hyperlipoproteinemias can be caused genetically (primary h.) or can occur in obesity and metabolic disorders (secondary h). Elevated LDL-cho- lesterol serum concentrations are associated with an increased risk of atherosclerosis, especially when there is a concomitant decline in HDL concentration (increase in LDL:HDL quotient).
Treatment. Various drugs are available that have different mechanisms of action and effects on LDL (cholesterol) and VLDL (triglycerides) (A). Their use is indicated in the therapy of primary hyperlipoproteinemias. In secondary hyperlipoproteinemias, the immediate goal should be to lower lipoprotein levels by dietary restriction, treatment of the primary disease, or both.
Drugs (B). Colestyramine and coles- tipol are nonabsorbable anion-exchange resins. By virtue of binding bile acids, they promote consumption of cholesterol for the synthesis of bile acids; the
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
|
Drugs used in Hyperlipoproteinemias 155 |
Dietary fats |
Cell metabolism |
|
Cholesterol |
LDL
Chylomicron |
Fat tissue |
|
Heart |
|
Skeletal muscle |
HDL
VLDL |
HDL |
LDL |
Chylomicron remnant
Cholesterol |
cell |
|
Liver |
||
Fatty acids |
||
Lipoprotein |
|
|
Lipase |
|
Lipoprotein |
Cholesterol |
synthesis |
|
|
Triglycerides |
Cholester
ester Triglycer
Cholesterol
Apolipo- |
OH |
|
OH |
protein |
OH |
||
|
|
|
A. Lipoprotein metabolism
Colestyramine |
|
|
Gut:: |
Bile acids |
Lipoproteins |
binding and |
|
|
excretion of |
|
cell |
bile acids (BA) |
|
|
Liver: |
|
Liver |
BA synthesis |
|
Cholesterol |
Cholesterol |
|
|
|
store |
|
consumption |
|
!-Sitosterol |
LDL |
Gut: |
|
Cholesterol |
Synthesis |
absorption |
|
|
HMG-CoA-Reductase inhibitors |
B. Cholesterol metabolism in liver cell and cholesterol-lowering drugs
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

156 Drugs used in Hyperlipoproteinemias
liver meets its increased cholesterol demand by enhancing the expression of HMG CoA reductase and LDL receptors (negative feedback).
At the required dosage, the resins cause diverse gastrointestinal disturbances. In addition, they interfere with the absorption of fats and fat-soluble vitamins (A, D, E, K). They also adsorb and decrease the absorption of such drugs as digitoxin, vitamin K antagonists, and diuretics. Their gritty texture and bulk make ingestion an unpleasant experience.
The statins, lovastatin (L), simvasta- tin (S), pravastatin (P), fluvastatin (F), cerivastatin, and atorvastatin, inhibit HMG CoA reductase. The active group of L, S, P, and F (or their metabolites) resembles that of the physiological substrate of the enzyme (A). L and S are lactones that are rapidly absorbed by the enteral route, subjected to extensive first-pass extraction in the liver, and there hydrolyzed into active metabolites. P and F represent the active form and, as acids, are actively transported by a specific anion carrier that moves bile acids from blood into liver and also mediates the selective hepatic uptake of the mycotoxin, amanitin (A). Atorvasta- tin has the longest duration of action. Normally viewed as presystemic elimination, efficient hepatic extraction serves to confine the action of the statins to the liver. Despite the inhibition of HMG CoA reductase, hepatic cholesterol content does not fall, because hepatocytes compensate any drop in cholesterol levels by increasing the synthesis of LDL receptor protein (along with the reductase). Because the newly formed reductase is inhibited, too, the hepatocyte must meet its cholesterol demand by uptake of LDL from the blood (B). Ac- cordingly, the concentration of circulating LDL decreases, while its hepatic clearance from plasma increases. There is also a decreased likelihood of LDL being oxidized into its proatheroslerotic degradation product. The combination of a statin with an ion-exchange resin intensifies the decrease in LDL levels. A
rare, but dangerous, side effect of the statins is damage to skeletal musculature. This risk is increased by combined use of fibric acid agents (see below).
Nicotinic acid and its derivatives
(pyridylcarbinol, xanthinol nicotinate, acipimox) activate endothelial lipoprotein lipase and thereby lower triglyceride levels. At the start of therapy, a prostaglandin-mediated vasodilation occurs (flushing and hypotension) that can be prevented by low doses of acetylsalicylic acid.
Clofibrate and derivatives (bezafibrate, etofibrate, gemfibrozil) lower plasma lipids by an unknown mechanism. They may damage the liver and skeletal muscle (myalgia, myopathy, rhabdomyolysis).
Probucol lowers HDL more than LDL; nonetheless, it appears effective in reducing atherogenesis, possibly by reducing LDL oxidation.
!3-Polyunsaturated fatty acids (eicosapentaenoate, docosahexaenoate) are abundant in fish oils. Dietary supplementation results in lowered levels of triglycerides, decreased synthesis of VLDL and apolipoprotein B, and improved clearance of remnant particles, although total and LDL cholesterol are not decreased or are even increased. High dietary intake may correlate with a reduced incidence of coronary heart disease.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Drugs used in Hyperlipoproteinemias 157
Low systemic availability
|
3-Hydroxy-3-methyl- |
Mevalonate |
|
|
|
|
glutaryl-CoA |
|
|
|
|
|
HMG-CoA |
|
|
|
|
|
Reductase |
Cholesterol |
|
|
|
|
Bio- |
|
|
|
|
|
activation |
|
|
|
|
|
|
Active form |
|
|
|
|
Extraction |
|
Active |
|
|
|
of lipophilic |
|
uptake of |
|
|
|
lactone |
|
anion |
|
|
HO |
O |
|
|
HO |
COOH |
|
|
|
|||
|
O |
|
|
|
OH |
|
|
|
|
|
|
O |
|
|
F |
|
CH3 |
|
|
Oral |
|
||
H3C |
O |
|
|
||
|
|
|
|||
H3C |
CH3 |
administration |
|
N CH3 |
|
H3C |
Lovastatin |
Fluvastatin |
|
|
|
|
|
|
|||
A. Accumulation and effect of HMG-CoA reductase inhibitors in liver
|
|
Inhibition of |
|
|
HMG-CoA reductase |
LDL- |
|
|
Receptor |
|
|
HMG-CoA |
Expression |
Expression |
reductase |
|
|
|
Cholesterol |
|
LDL |
|
Increased receptor- |
|
mediated uptake of LDL |
|
in blood |
|
|
B.Regulation by cellular cholesterol concentration of HMG-CoA reductase and LDL-receptors
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

158 Diuretics
Diuretics – An Overview
Diuretics (saluretics) elicit increased production of urine (diuresis). In the strict sense, the term is applied to drugs with a direct renal action. The predominant action of such agents is to augment urine excretion by inhibiting the reabsorption of NaCl and water.
The most important indications for diuretics are:
Mobilization of edemas (A): In edema there is swelling of tissues due to accumulation of fluid, chiefly in the extracellular (interstitial) space. When a diuretic is given, increased renal excretion of Na+ and H2O causes a reduction in plasma volume with hemoconcentration. As a result, plasma protein concentration rises along with oncotic pressure. As the latter operates to attract water, fluid will shift from interstitium into the capillary bed. The fluid content of tissues thus falls and the edemas recede. The decrease in plasma volume and interstitial volume means a diminution of the extracellular fluid volume (EFV). Depending on the condition, use is made of: thiazides, loop diuretics, aldosterone antagonists, and osmotic diuretics.
Antihypertensive therapy. Diuretics have long been used as drugs of first choice for lowering elevated blood pressure (p. 312). Even at low dosage, they decrease peripheral resistance (without significantly reducing EFV) and thereby normalize blood pressure.
Therapy of congestive heart failure.
By lowering peripheral resistance, diuretics aid the heart in ejecting blood (reduction in afterload, pp. 132, 306); cardiac output and exercise tolerance are increased. Due to the increased excretion of fluid, EFV and venous return decrease (reduction in preload, p. 306). Symptoms of venous congestion, such as ankle edema and hepatic enlargement, subside. The drugs principally used are thiazides (possibly combined with K+-sparing diuretics) and loop diuretics.
Prophylaxis of renal failure. In circulatory failure (shock), e.g., secondary to massive hemorrhage, renal production of urine may cease (anuria). By means of diuretics an attempt is made to maintain urinary flow. Use of either osmotic or loop diuretics is indicated.
Massive use of diuretics entails a hazard of adverse effects (A): (1) the decrease in blood volume can lead to hypotension and collapse; (2) blood viscosity rises due to the increase in erythro- and thrombocyte concentration, bringing an increased risk of intravascular coagulation or thrombosis.
When depletion of NaCl and water (EFV reduction) occurs as a result of diuretic therapy, the body can initiate counter-regulatory responses (B), namely, activation of the renin-angio- tensin-aldosterone system (p. 124). Because of the diminished blood volume, renal blood flow is jeopardized. This leads to release from the kidneys of the hormone, renin, which enzymatically catalyzes the formation of angiotensin I. Angiotensin I is converted to angiotensin II by the action of angiotensin-con- verting enzyme (ACE). Angiotensin II stimulates release of aldosterone. The mineralocorticoid promotes renal reabsorption of NaCl and water and thus counteracts the effect of diuretics. ACE inhibitors (p. 124) augment the effectiveness of diuretics by preventing this counter-regulatory response.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
Diuretics |
159 |
|
Protein molecules |
|
|
Edema |
|
|
|
|
Hemoconcentration |
|
|
|
Colloid |
|
|
|
osmotic |
|
|
|
pressure |
|
|
Mobilization of |
Collapse, |
|
|
danger of |
|
|
|
edema fluid |
thrombosis |
Diuretic |
|
A. Mechanism of edema fluid mobilization by diuretics
Salt and |
Diuretic |
Diuretic |
|
fluid retention |
|||
|
|
||
EFV: |
|
|
|
Na+, Cl-, |
|
|
|
H2O |
|
|
|
|
Angiotensinogen |
|
|
|
Renin |
|
|
|
Angiotensin I |
|
|
|
ACE |
|
|
|
Angiotensin II |
Aldosterone |
B. Possible counter-regulatory responses during long-term diuretic therapy
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

160 Diuretics
NaCl Reabsorption in the Kidney (A)
The smallest functional unit of the kidney is the nephron. In the glomerular capillary loops, ultrafiltration of plasma fluid into Bowman’s capsule (BC) yields primary urine. In the proximal tubules (pT), approx. 70% of the ultrafiltrate is retrieved by isoosmotic reabsorption of NaCl and water. In the thick portion of the ascending limb of Henle’s loop (HL), NaCl is absorbed unaccompanied by water. This is the prerequisite for the hairpin countercurrent mechanism that allows build-up of a very high NaCl concentration in the renal medulla. In the distal tubules (dT), NaCl and water are again jointly reabsorbed. At the end of the nephron, this process involves an al- dosterone-controlled exchange of Na+ against K+ or H+. In the collecting tubule (C), vasopressin (antidiuretic hormone, ADH) increases the epithelial permeability for water, which is drawn into the hyperosmolar milieu of the renal medulla and thus retained in the body. As a result, a concentrated urine enters the renal pelvis.
Na+ transport through the tubular cells basically occurs in similar fashion in all segments of the nephron. The intracellular concentration of Na+ is significantly below that in primary urine. This concentration gradient is the driving force for entry of Na+ into the cytosol of tubular cells. A carrier mechanism moves Na+ across the membrane. Energy liberated during this influx can be utilized for the coupled outward transport of another particle against a gradient. From the cell interior, Na+ is moved with expenditure of energy (ATP hydrolysis) by Na+/K+-ATPase into the extracellular space. The enzyme molecules are confined to the basolateral parts of the cell membrane, facing the interstitium; Na+ can, therefore, not escape back into tubular fluid.
All diuretics inhibit Na+ reabsorption. Basically, either the inward or the outward transport of Na+ can be affected.
Osmotic Diuretics (B)
Agents: mannitol, sorbitol. Site of action: mainly the proximal tubules. Mode of action: Since NaCl and H2O are reabsorbed together in the proximal tubules, Na+ concentration in the tubular fluid does not change despite the extensive reabsorption of Na+ and H2O. Body cells lack transport mechanisms for polyhydric alcohols such as mannitol (structure on p. 171) and sorbitol, which are thus prevented from penetrating cell membranes. Therefore, they need to be given by intravenous infusion. They also cannot be reabsorbed from the tubular fluid after glomerular filtration. These agents bind water osmotically and retain it in the tubular lumen. When Na ions are taken up into the tubule cell, water cannot follow in the usual amount. The fall in urine Na+ concentration reduces Na+ reabsorption, in part because the reduced concentration gradient towards the interior of tubule cells means a reduced driving force for Na+ influx. The result of osmotic diuresis is a large volume of dilute urine.
Indications: prophylaxis of renal hypovolemic failure, mobilization of brain edema, and acute glaucoma.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
