
книги студ / Color Atlas of Pharmacology
.pdf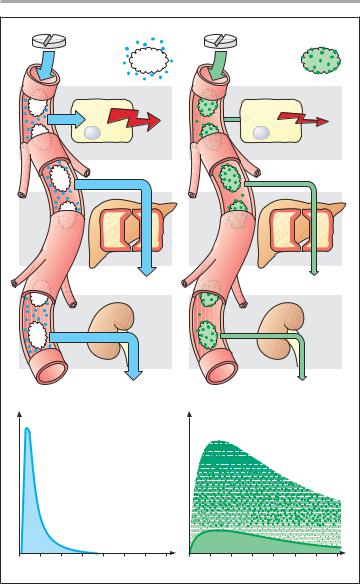
|
|
Distribution in the Body |
31 |
|
|
Drug is |
|
Drug is |
|
|
not bound |
|
strongly |
|
|
to plasma |
|
bound to |
|
|
proteins |
|
plasma |
|
|
|
|
proteins |
|
|
Effect |
|
Effect |
|
|
fector cell |
|
fector cell |
|
|
Biotransformation |
|
Biotransformation |
|
|
Renal elimination |
|
Renal elimination |
|
Plasma |
concentration |
Plasma |
concentration |
|
|
Free drug |
Bound drug |
|
|
|
|
|
||
|
|
Free drug |
|
|
|
Time |
|
Time |
|
A. Importance of protein binding for intensity and duration of drug effect
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

32 Drug Elimination
The Liver as an Excretory Organ
As the chief organ of drug biotransformation, the liver is richly supplied with blood, of which 1100 mL is received each minute from the intestines through the portal vein and 350 mL through the hepatic artery, comprising nearly 1/3 of cardiac output. The blood content of hepatic vessels and sinusoids amounts to 500 mL. Due to the widening of the portal lumen, intrahepatic blood flow decelerates (A). Moreover, the endothelial lining of hepatic sinusoids (p. 24) contains pores large enough to permit rapid exit of plasma proteins. Thus, blood and hepatic parenchyma are able to maintain intimate contact and intensive exchange of substances, which is further facilitated by microvilli covering the hepatocyte surfaces abutting Disse’s spaces.
The hepatocyte secretes biliary fluid into the bile canaliculi (dark green), tubular intercellular clefts that are sealed off from the blood spaces by tight junctions. Secretory activity in the hepatocytes results in movement of fluid towards the canalicular space (A). The hepatocyte has an abundance of enzymes carrying out metabolic functions. These are localized in part in mitochondria, in part on the membranes of the rough (rER) or smooth (sER) endoplasmic reticulum.
Enzymes of the sER play a most important role in drug biotransformation. At this site, molecular oxygen is used in oxidative reactions. Because these enzymes can catalyze either hydroxylation or oxidative cleavage of -N-C- or -O-C- bonds, they are referred to as “mixedfunction” oxidases or hydroxylases. The essential component of this enzyme system is cytochrome P450, which in its oxidized state binds drug substrates (R- H). The FeIII-P450-RH binary complex is first reduced by NADPH, then forms the ternary complex, O2-FeII-P450-RH, which accepts a second electron and finally disintegrates into FeIII-P450, one equivalent of H2O, and hydroxylated drug (R-OH).
Compared with hydrophilic drugs not undergoing transport, lipophilic drugs are more rapidly taken up from the blood into hepatocytes and more readily gain access to mixed-function oxidases embedded in sER membranes. For instance, a drug having lipophilicity by virtue of an aromatic substituent (phenyl ring) (B) can be hydroxylated and, thus, become more hydrophilic (Phase I reaction, p. 34). Besides oxidases, sER also contains reductases and glucuronyl transferases. The latter conjugate glucuronic acid with hydroxyl, carboxyl, amine, and amide groups (p. 38); hence, also phenolic products of phase I metabolism (Phase II conjugation). Phase I and Phase II metabolites can be transported back into the blood
— probably via a gradient-dependent carrier — or actively secreted into bile.
Prolonged exposure to certain substrates, such as phenobarbital, carbamazepine, rifampicin results in a proliferation of sER membranes (cf. C and D). This enzyme induction, a load-depen- dent hypertrophy, affects equally all enzymes localized on sER membranes. Enzyme induction leads to accelerated biotransformation, not only of the inducing agent but also of other drugs (a form of drug interaction). With continued exposure, induction develops in a few days, resulting in an increase in reaction velocity, maximally 2–3fold, that disappears after removal of the inducing agent.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
Drug Elimination |
33 |
Hepatocyte |
Biliary capillary |
Disse´s space |
|
|
|
|
Intestine |
|
Gall-bladder |
|
|
A. Flow patterns in portal vein, Disse’s space, and hepatocyte |
|
||
|
Phase I- |
|
|
|
metabolite |
sER |
|
|
|
|
|
|
Biliary |
rER |
|
|
|
|
|
|
capillary |
|
|
|
|
C. Normal hepatocyte |
|
|
Phase II- |
|
|
|
metabolite |
|
|
|
Glucuronide |
|
|
Carrier |
|
rER |
|
|
|
|
|
|
|
sER |
|
B. Fate of drugs undergoing |
D. Hepatocyte after |
B. hepatic hydroxylation |
D. phenobarbital administration |
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

34 Drug Elimination
Biotransformation of Drugs
Many drugs undergo chemical modification in the body (biotransformation). Most frequently, this process entails a loss of biological activity and an increase in hydrophilicity (water solubility), thereby promoting elimination via the renal route (p. 40). Since rapid drug elimination improves accuracy in titrating the therapeutic concentration, drugs are often designed with built-in weak links. Ester bonds are such links, being subject to hydrolysis by the ubiquitous esterases. Hydrolytic cleavages, along with oxidations, reductions, alkylations, and dealkylations, constitute Phase I reactions of drug metabolism. These reactions subsume all metabolic processes apt to alter drug molecules chemically and take place chiefly in the liver. In
Phase II (synthetic) reactions, conjugation products of either the drug itself or its Phase I metabolites are formed, for instance, with glucuronic or sulfuric acid (p. 38).
The special case of the endogenous transmitter acetylcholine illustrates well the high velocity of ester hydrolysis. Acetylcholine is broken down at its sites of release and action by acetylcholinesterase (pp. 100, 102) so rapidly as to negate its therapeutic use. Hydrolysis of other esters catalyzed by various esterases is slower, though relatively fast in comparison with other biotransformations. The local anesthetic, procaine, is a case in point; it exerts its action at the site of application while being largely devoid of undesirable effects at other locations because it is inactivated by hydrolysis during absorption from its site of application.
Ester hydrolysis does not invariably lead to inactive metabolites, as exemplified by acetylsalicylic acid. The cleavage product, salicylic acid, retains pharmacological activity. In certain cases, drugs are administered in the form of esters in order to facilitate absorption (enalapril ! enalaprilate; testosterone undecanoate ! testosterone) or to reduce irritation of the gastrointestinal
mucosa (erythromycin succinate ! erythromycin). In these cases, the ester itself is not active, but the cleavage product is. Thus, an inactive precursor or prodrug is applied, formation of the active molecule occurring only after hydrolysis in the blood.
Some drugs possessing amide bonds, such as prilocaine, and of course, peptides, can be hydrolyzed by peptidases and inactivated in this manner. Peptidases are also of pharmacological interest because they are responsible for the formation of highly reactive cleavage products (fibrin, p. 146) and potent mediators (angiotensin II, p. 124; bradykinin, enkephalin, p. 210) from biologically inactive peptides.
Peptidases exhibit some substrate selectivity and can be selectively inhibited, as exemplified by the formation of angiotensin II, whose actions inter alia include vasoconstriction. Angiotensin II is formed from angiotensin I by cleavage of the C-terminal dipeptide histidylleucine. Hydrolysis is catalyzed by “angio- tensin-converting enzyme” (ACE). Peptide analogues such as captopril (p. 124) block this enzyme. Angiotensin II is degraded by angiotensinase A, which clips off the N-terminal asparagine residue. The product, angiotensin III, lacks vasoconstrictor activity.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
|
Drug Elimination |
|
35 |
|||
Esterases |
Ester |
Peptidases |
Amides |
Anilides |
|
|
||
Acetylcholine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Converting |
|
|
|
|
|
|
|
|
enzyme |
|
|
|
|
Angiotensin |
I |
|
|
|
|
|
Acetic acid |
|
|
|
|
|
|
|
|
|
|
|
Angiotensin |
II |
|
|
|
|
|
|
|
|
|
III |
|
||
|
Choline |
|
|
|
Angiotensin |
|
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
Procaine |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
p-Aminobenzoic acid |
|
|
|
|
|
|
|
|
Diethylaminoethanol |
|
|
|
|
|
|
|
|
Acetylsalicylic acid |
|
|
Prilocaine |
|
|
|||
Acetic acid |
Salicylic acid |
N-Propylalanine |
|
Toluidine |
|
|||
A. Examples of chemical reactions in drug biotransformation (hydrolysis)
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

36 Drug Elimination
Oxidation reactions can be divided into two kinds: those in which oxygen is incorporated into the drug molecule, and those in which primary oxidation causes part of the molecule to be lost. The former include hydroxylations, epoxidations, and sulfoxidations. Hydroxylations may involve alkyl substituents (e.g., pentobarbital) or aromatic ring systems (e.g., propranolol). In both cases, products are formed that are conjugated to an organic acid residue, e.g., glucuronic acid, in a subsequent Phase II reaction.
Hydroxylation may also take place at nitrogen atoms, resulting in hydroxylamines (e.g., acetaminophen). Benzene, polycyclic aromatic compounds (e.g., benzopyrene), and unsaturated cyclic carbohydrates can be converted by mono-oxygenases to epoxides, highly reactive electrophiles that are hepatotoxic and possibly carcinogenic.
The second type of oxidative biotransformation comprises dealkyla- tions. In the case of primary or secondary amines, dealkylation of an alkyl group starts at the carbon adjacent to the nitrogen; in the case of tertiary amines, with hydroxylation of the nitrogen (e.g., lidocaine). The intermediary products are labile and break up into the dealkylated amine and aldehyde of the alkyl group removed. O-dealkylation
O2
R1
N
R2
CH3  R1
R1
and S-dearylation proceed via an analogous mechanism (e.g., phenacetin and azathioprine, respectively).
Oxidative deamination basically resembles the dealkylation of tertiary amines, beginning with the formation of a hydroxylamine that then decomposes into ammonia and the corresponding aldehyde. The latter is partly reduced to an alcohol and partly oxidized to a carboxylic acid.
Reduction reactions may occur at oxygen or nitrogen atoms. Keto-oxy- gens are converted into a hydroxyl group, as in the reduction of the prodrugs cortisone and prednisone to the active glucocorticoids cortisol and prednisolone, respectively. N-reductions occur in azoor nitro-compounds (e.g., nitrazepam). Nitro groups can be reduced to amine groups via nitroso and hydroxylamino intermediates. Likewise, dehalogenation is a reductive process involving a carbon atom (e.g., halothane, p. 218).
Methylations are catalyzed by a family of relatively specific methyltransferases involving the transfer of methyl groups to hydroxyl groups (O- methylation as in norepinephrine [noradrenaline]) or to amino groups (N- methylation of norepinephrine, histamine, or serotonin).
In thio compounds, desulfuration
N |
OH |
|
|
|
R2 |
|
|
|
|
|
CH3 |
|
|
|
Desalkylierung |
R1 |
+ |
|
O |
|
|
H C |
||
|
N |
|
|
CH3 |
|
R2 |
H |
|
|
L llmann, Color Atlas of Pharmacology ' 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
Drug Elimination |
37 |
|
|
Hydroxylation |
|
|
|
Lidocaine |
Phenacetin |
|
Parathion |
|
N-Dealkylation |
|
|
|
|
|
O-Dealkylation |
|
Desulfuration |
|
|
|
|
|
|
|
Dealkylation |
|
Norepinephrine |
|
|
|
|
|
|
|
S-Dealkylation |
|
|
|
|
O-Methylation |
|
||
Azathioprine |
|
|
Methylation |
|
Nitrazepam |
Benzpyrene |
Chlorpromazine |
|
|
|
|
|
Acetaminophen |
|
|
|
Sulfoxidation |
|
|
|
Epoxidation |
|
|
|
|
|
|
Hydroxyl- |
|
Reduction |
Oxidation |
amine |
|
|
|
|
|||
A. Examples of chemical reactions in drug biotransformation
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

38 Drug Elimination
Enterohepatic Cycle (A)
After an orally ingested drug has been absorbed from the gut, it is transported via the portal blood to the liver, where it can be conjugated to glucuronic or sulfuric acid (shown in B for salicylic acid and deacetylated bisacodyl, respectively) or to other organic acids. At the pH of body fluids, these acids are predominantly ionized; the negative charge confers high polarity upon the conjugated drug molecule and, hence, low membrane penetrability. The conjugated products may pass from hepatocyte into biliary fluid and from there back into the intestine. O-glucuronides can be cleaved by bacterial !-glucuronidases in the colon, enabling the liberated drug molecule to be reabsorbed. The entero- hepatic cycle acts to trap drugs in the body. However, conjugated products enter not only the bile but also the blood. Glucuronides with a molecular weight (MW) > 300 preferentially pass into the blood, while those with MW > 300 enter the bile to a larger extent. Glucuronides circulating in the blood undergo glomerular filtration in the kidney and are excreted in urine because their decreased lipophilicity prevents tubular reabsorption.
Drugs that are subject to enterohepatic cycling are, therefore, excreted slowly. Pertinent examples include digitoxin and acidic nonsteroidal anti-in- flammatory agents (p. 200).
Conjugations (B)
The most important of phase II conjugation reactions is glucuronidation. This reaction does not proceed spontaneously, but requires the activated form of glucuronic acid, namely glucuronic acid uridine diphosphate. Microsomal glucuronyl transferases link the activated glucuronic acid with an acceptor molecule. When the latter is a phenol or alcohol, an ether glucuronide will be formed. In the case of carboxyl-bearing molecules, an ester glucuronide is the result. All of these are O-glucuronides.
Amines may form N-glucuronides that, unlike O-glucuronides, are resistant to bacterial !-glucuronidases.
Soluble cytoplasmic sulfotransferases conjugate activated sulfate (3’- phosphoadenine-5’-phosphosulfate) with alcohols and phenols. The conjugates are acids, as in the case of glucuronides. In this respect, they differ from conjugates formed by acetyltransferases from activated acetate (acetylcoenzyme A) and an alcohol or a phenol.
Acyltransferases are involved in the conjugation of the amino acids glycine or glutamine with carboxylic acids. In these cases, an amide bond is formed between the carboxyl groups of the acceptor and the amino group of the donor molecule (e.g., formation of salicyluric acid from salicylic acid and glycine). The acidic group of glycine or glutamine remains free.
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
|
Drug Elimination |
39 |
|
|
|
|
|
1 |
|
Hepatocyte |
|
|
|
|
|
Sinusoid |
|
|
|
|
|
Biliary capillary |
4 |
|
|
Biliary |
|
|
|
5 |
|
||
|
Conjugation with |
elimination |
|
||
|
3 |
|
|
||
7 |
|
|
|
||
glucuronic acid |
|
|
|
||
|
|
|
|
||
|
|
|
2 |
|
|
|
|
|
|
|
|
Portal vein
E nte
ro
h e
p
a t i
c
c
i
|
|
|
|
|
n |
|
|
|
|
o |
|
|
|
|
i |
|
|
|
|
t |
|
|
|
|
la |
|
|
|
|
u |
|
|
|
|
|
rc |
|
|
|
|
|
Deconjugation
6by microbial !-glucuronidase
8 |
Renal |
Lipophilic |
Enteral |
|
elimination |
drug |
absorption |
||
|
||||
|
|
Hydrophilic |
|
|
|
|
conjugation product |
||
A. Enterohepatic cycle |
|
|
||
UDP- -Glucuronic acid |
|
3'-Phosphoadenine-5'-phosphosulfate |
||
Glucuronyl- |
Sulfo- |
transferase |
transferase |
Salicylic acid |
Active moiety of bisacodyl |
B. Conjugation reactions
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

40 Drug Elimination
The Kidney as Excretory Organ
Most drugs are eliminated in urine either chemically unchanged or as metabolites. The kidney permits elimination because the vascular wall structure in the region of the glomerular capillaries (B) allows unimpeded passage of blood solutes having molecular weights (MW) < 5000. Filtration diminishes progressively as MW increases from 5000 to 70000 and ceases at MW > 70000. With few exceptions, therapeutically used drugs and their metabolites have much smaller molecular weights and can, therefore, undergo glomerular filtration, i.e., pass from blood into primary urine. Separating the capillary endothe- lium from the tubular epithelium, the basal membrane consists of charged glycoproteins and acts as a filtration barrier for high-molecular-weight substances. The relative density of this barrier depends on the electrical charge of molecules that attempt to permeate it.
Apart from glomerular filtration
(B), drugs present in blood may pass into urine by active secretion. Certain cations and anions are secreted by the epithelium of the proximal tubules into the tubular fluid via special, energyconsuming transport systems. These transport systems have a limited capacity. When several substrates are present simultaneously, competition for the carrier may occur (see p. 268).
During passage down the renal tubule, urinary volume shrinks more than 100-fold; accordingly, there is a corresponding concentration of filtered drug or drug metabolites (A). The resulting concentration gradient between urine and interstitial fluid is preserved in the case of drugs incapable of permeating the tubular epithelium. However, with lipophilic drugs the concentration gradient will favor reabsorption of the filtered molecules. In this case, reabsorption is not based on an active process but results instead from passive diffusion. Accordingly, for protonated substances, the extent of reabsorption is dependent upon urinary pH or the de-
gree of dissociation. The degree of dissociation varies as a function of the urinary pH and the pKa, which represents the pH value at which half of the substance exists in protonated (or unprotonated) form. This relationship is graphically illustrated (D) with the example of a protonated amine having a pKa of 7.0. In this case, at urinary pH 7.0, 50 % of the amine will be present in the protonated, hydrophilic, membrane-impermeant form (blue dots), whereas the other half, representing the uncharged amine (orange dots), can leave the tubular lumen in accordance with the resulting concentration gradient. If the pKa of an amine is higher (pKa = 7.5) or lower (pKa = 6.5), a correspondingly smaller or larger proportion of the amine will be present in the uncharged, reabsorbable form. Lowering or raising urinary pH by half a pH unit would result in analogous changes for an amine having a pKa of 7.0.
The same considerations hold for acidic molecules, with the important difference that alkalinization of the urine (increased pH) will promote the deprotonization of -COOH groups and thus impede reabsorption. Intentional alteration in urinary pH can be used in intoxications with proton-acceptor substances in order to hasten elimination of the toxin (alkalinization ! phenobarbital; acidification ! amphetamine).
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
