
- •Chrome subgroup physical properties
- •Chrome subgroup trends
- •History Of Discovery
- •Occurrence
- •Preparation
- •Electronic Configurations & Oxidation States
- •Chemical Properties. Free Chrome And Compounds (0)
- •Low oxidation states
- •Compounds e(IV)
- •Compounds e(V)
- •Questions and tasks
- •Make up the equations o f the reactions Make up the equations of the reactions
- •Experimental section
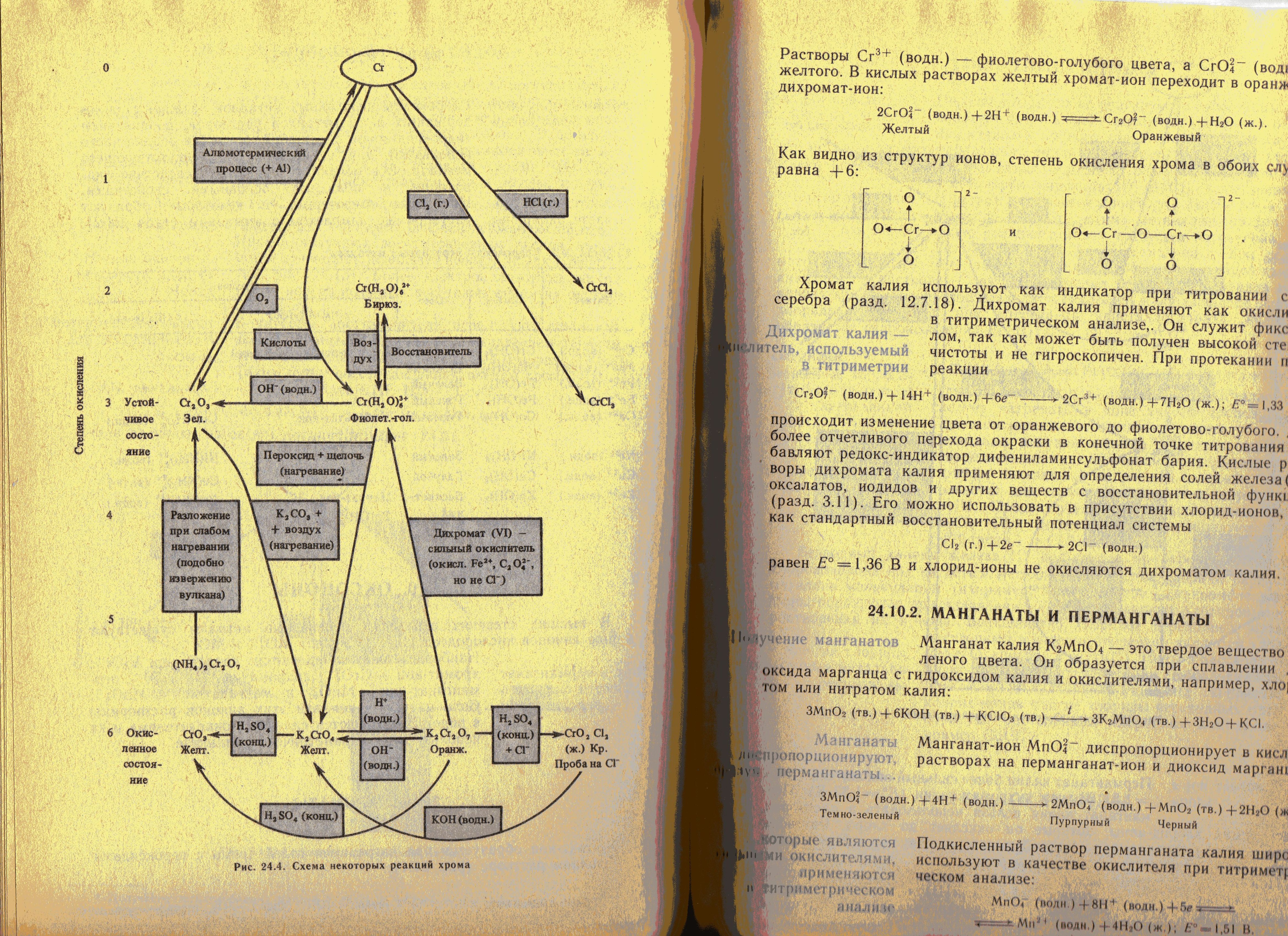
- •2. Chemical properties of chrome
- •3. Chemical properties to molybdenum and tungsten
- •Chrome. Standard electrode potentials, eo, V
- •Mendeleev's predicted elements
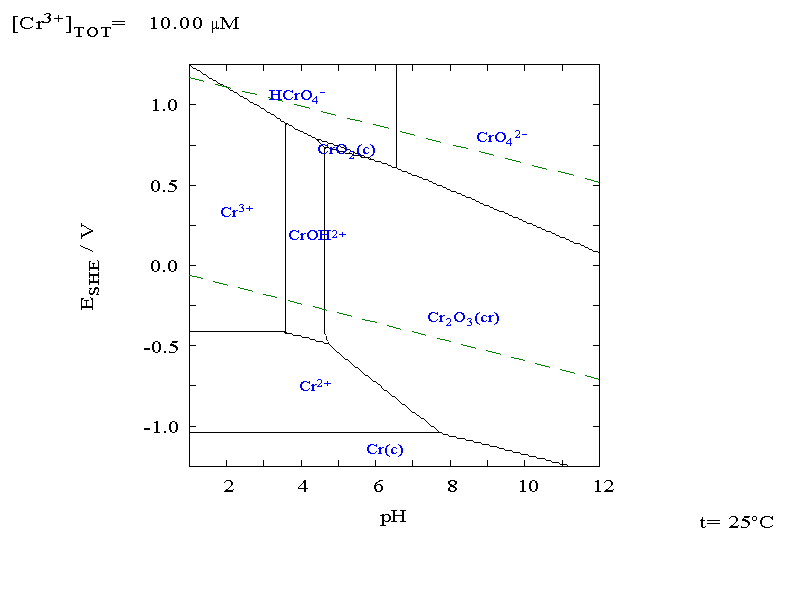
- •The Pourbaix diagram for chrome in pure water, perchloric acid or sodium hydroxide
- •M olybdenum Wheels
- •From Recent Developments in Inorganic Chemistry 2005
3. Chemical properties to molybdenum and tungsten
3.1. Place 2-3 mls of 0,5N ammonium molybdate solution into a test tube, add 3-4 drops of concentrated hydrochloric acid and 1-2 granules of metallic zinc. What occurs? Give the equations of the relevant reactions, taking into account, that the following compounds are formed: (NH4)2[MoOCl5] is green, and (NH4)4[MoOCl4] is brown.
3.2. Place 2-3 mls of 0,5N ammonium molybdate solution into a test tube and add by drops diluted nitric acid, avoiding its excess amount, until precipitate forms. Divide the precipitate in two parts in two test tubes and add excess amount of 1 M hydrochloric acid solution to one of them, and excess amount of 1 M sodium hydroxide solution to the second. What occurs? Give the equations of the relevant reactions and make a conclusion on acid-base properties of compound.
3.3. Place 2-3 mls of 0,5N ammonium tungstenate solution into a test tube and add by drops diluted nitric acid, avoiding its excess amount, until precipitate forms. Divide the precipitate in two parts in two test tubes and add excess amount of 1 M hydrochloric acid solution to one of them, and excess amount of 1 M sodium hydroxide solution to the second. What occurs? Give the equations of the relevant reactions and make a conclusion on acid-base properties of compound.
Chrome. Standard electrode potentials, eo, V
VI V IV III II 0
Acid medium
 0,
95 - 0, 74
0,
95 - 0, 74
0, 55 1, 34 2, 10 - 0, 424 - 0, 90
Cr![]() O
O![]() CrO
CrO![]() Cr(IV) Cr
Cr(IV) Cr![]() Cr
Cr![]() Cr
Cr
1, 72
1, 38
Alkaline medium
- 0, 11 - 1, 33
 CrO
CrO![]() Cr(OH)
Cr(OH)![]() Cr
Cr
- 0, 72 - 1, 33
Cr(OH)![]()
Mendeleev's predicted elements
To give provisional names to these predicted elements, Mendeleev used the prefixes eka-, dvi -, and tri-, from the Sanskrit words for one, two, and three, depending upon whether the predicted element was one, two, or three places away from the known element in his table with similar chemical properties.
The Pourbaix diagram for chrome in pure water, perchloric acid or sodium hydroxide
green
yellow









![]()

![]()

![]()

![]()


M olybdenum Wheels
Soluble “molybdenum blues” have been known for over two hundred years, but it was not until recently that their structures were finally elucidated. They are giant clusters of molybdenum oxide typically containing between 100 and 360 molybdenum atoms. Interestingly, the clusters are often wheel-shaped with a nanometer-sized cavity thus having the fascinating possibility of host-guest chemistry.
Some Questions to consider: How are the solutions of molybdenum blue prepared and how are their structures determined? What are the building blocks of the resulting clusters and how do the basic units combine to give these architectures? What are the possible uses of these molecules?
Muller and Serain, Acc Chem. Res., 2000, 33, 2.
Muller et al, Angew. Chemie, Int. Ed. 2002, 41, 1162.
Muller et al, Angew. Chemie, Int. Ed. 2002, 41, 2805.
From Recent Developments in Inorganic Chemistry 2005

