
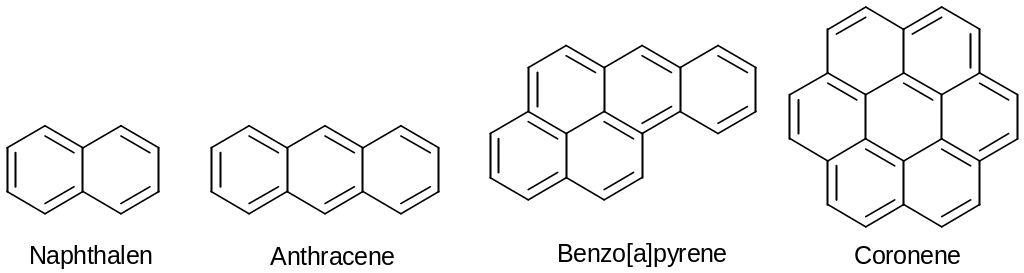
Polycyclic Aromatic Compound
Naphthalene: A Polycyclic Aromatic Compound
The Húckel rule is strictly applicable only to monocyclic aromatic compounds, but the general concept of aromaticity can be extended beyond simple monocyclic compounds ti include polycyclic aromatic compounds:
Nomenclature of Alkynes
N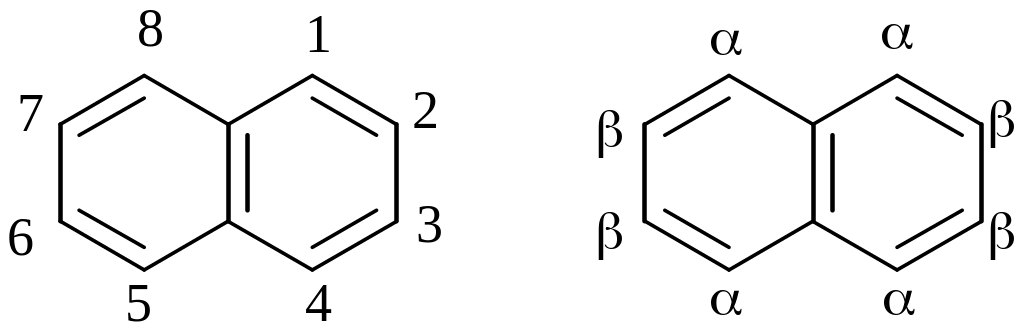 umber
the parent chain:
umber
the parent chain:
All polycyclic aromatic compounds can be represented by a number of different resonance forms. The true structure of naphthalene is a hybrid of the three resonance forms:
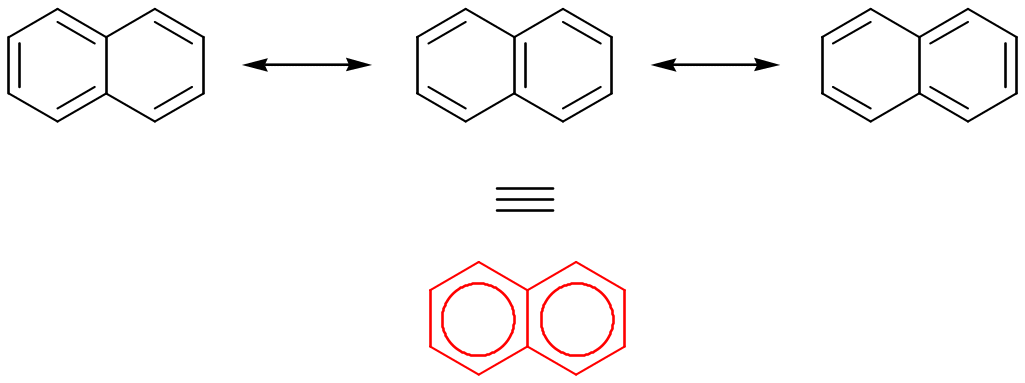
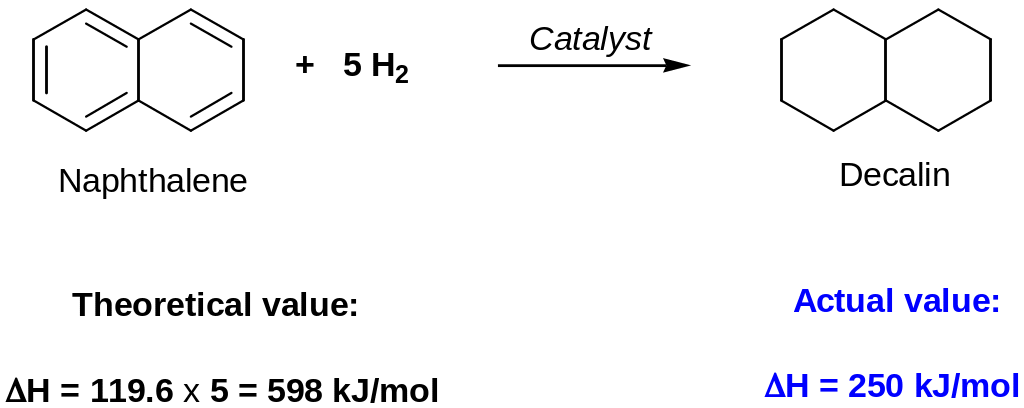
Naphthalene and other polycyclic aromatic compounds show many of the chemical properties associated with aromaticity.
Thus, heat of hydrogenation measurements show an aromatic stabilization:
Electrophilic Substitution Reactions
Naphthalene reacts with electrophiles to give, as a rule, 1-substituted products:
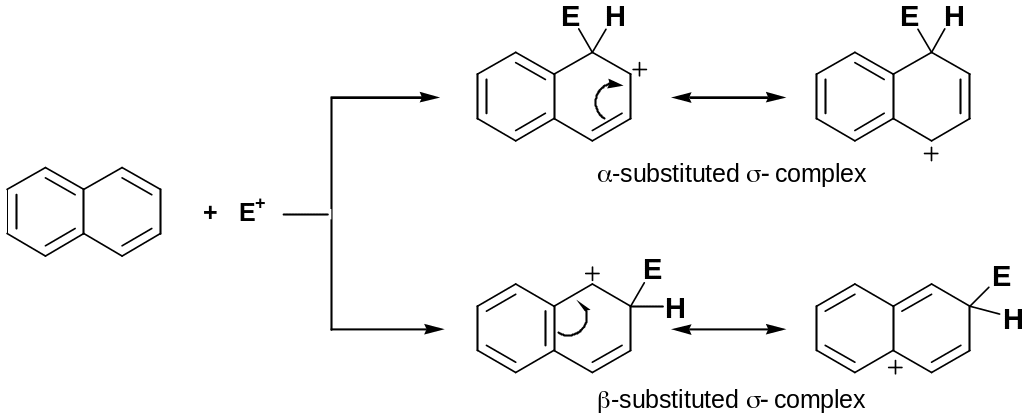
α-substituted σ-complex is more stable because during delocalization of positive charge one aromatic ring stay constant
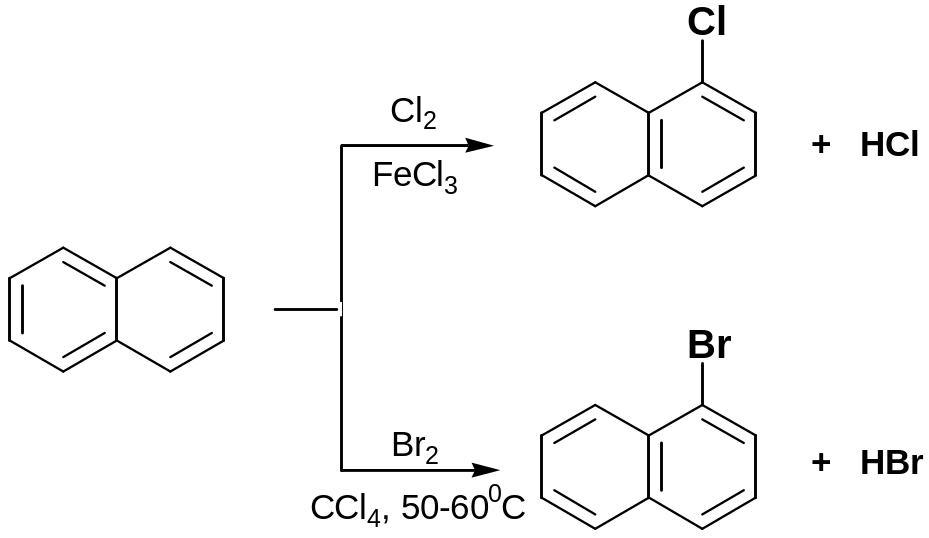
Halogenation
Naphtalene reacts with chlorine and bromine in the presence of catalysts such as AlCl3, FeCl3, CCl4, to give α-chloronaphthalene and α-bromonaphthalene respectively
Nitration
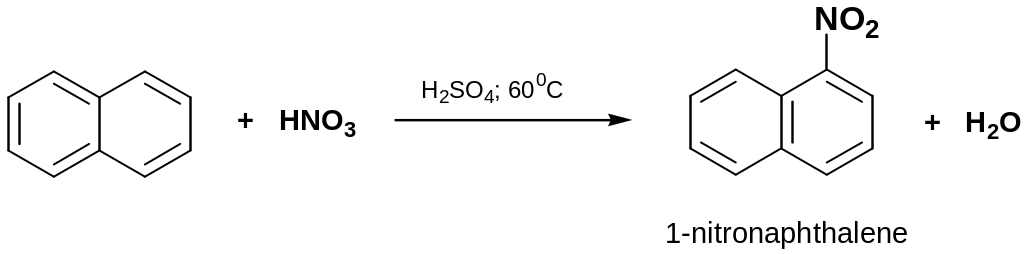
Conc. H2SO4 increases the rate of reaction by increasing the concentration of the electrophile, NO2+ (nitronium ion)
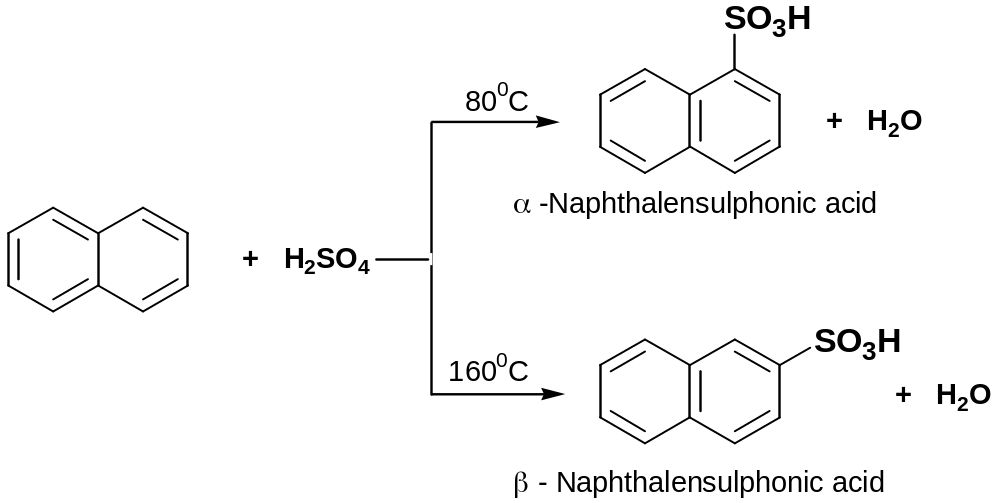
Sulphonation
Naphthalene reacts with fuming sulphuric(VI) acid at temperature 800C to give α-Naphthalenesulphonic acid
Heating above 160°C, β-Naphthalenesulphonic acid are formed
Addition Reactions
Naphthalene can undergo addition reaction easier than benzene:
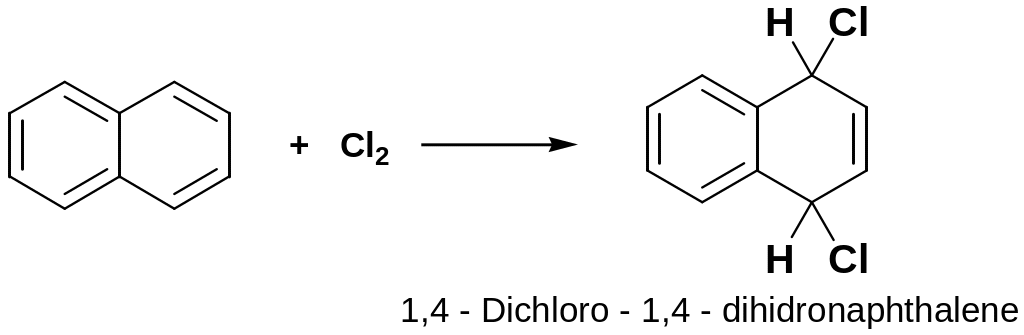
Addition of X2
Addition products with Cl2 are formed at room temperature with out catalyst
Redaction of naphthalene
Naphthalene adds hydrogen easier than benzene in the presence of catalysts such as Ni:

Substituent Effect in Substitututed Naphthalene
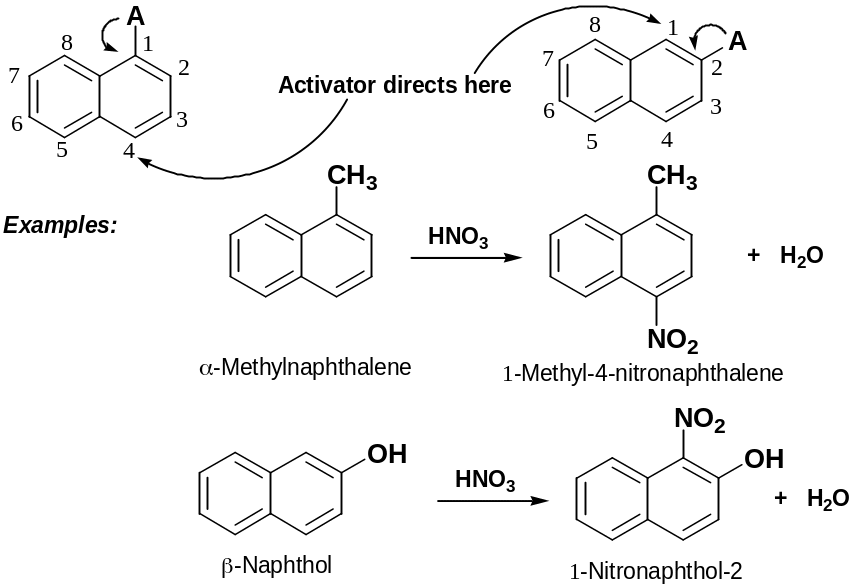
Directing effects of ortho- and para-directing activators
Directing effects of deactivators:
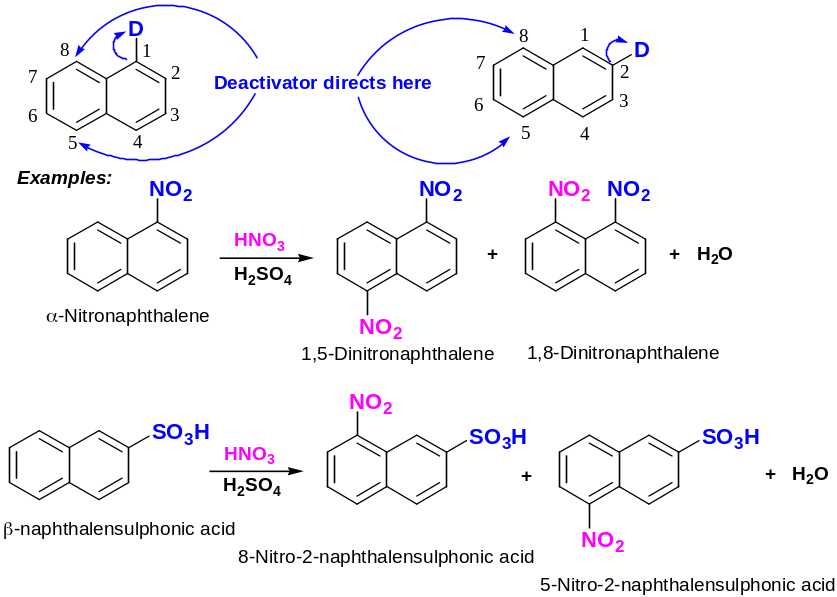
Reaction of sulphonation at high temperature is special:

Questions and problems
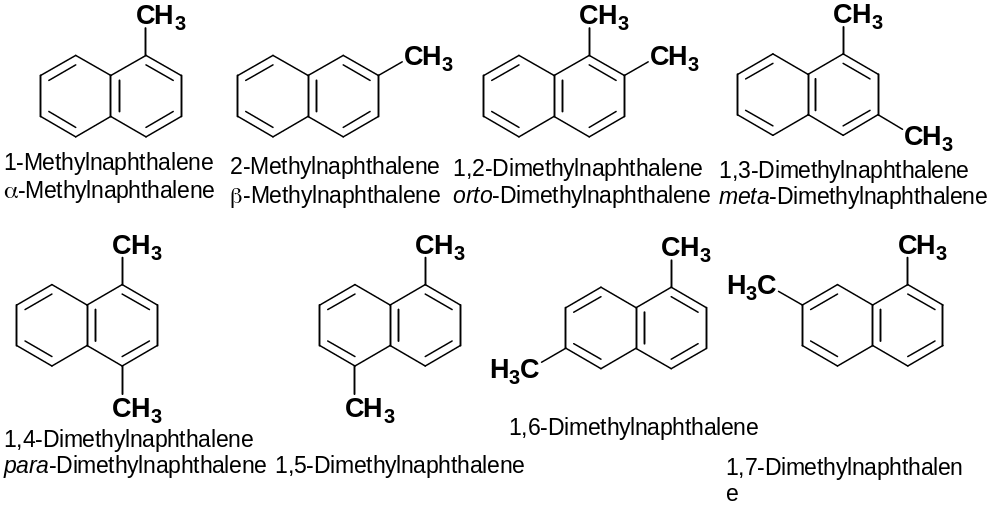
the name to the following compound: A) 3,5,7-trimethylnaphtalene; B) 2,4,6- trimethylnaphtalene; C) 1,3,7- trimethylnaphtalene; D) 3,5,8-trimethylbicyklo[4.4.0]decane.
Choose the right way of synthesis of nitrotertbutylbenzene. Write the equations. А)
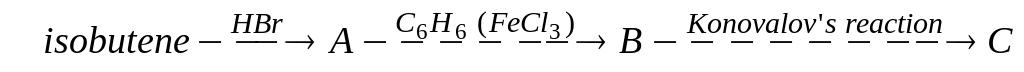 ;
В)
;
В)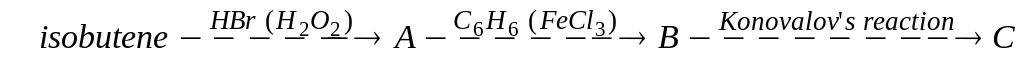 ;
С)
;
С)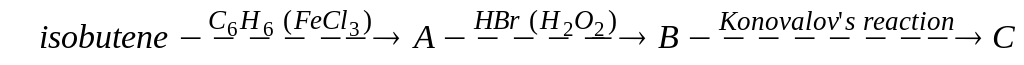 ;
D)
;
D)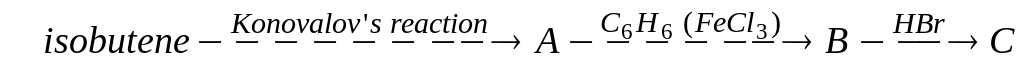 .
.What is the product of toluene alkilation by 1-chloro-2-methylpropane (AlCl3 present) Write the equations. A) 1-tertbutyl-3-methylbenzene; B) 1-secbutyl-3- methylbenzene; C) 1-methyl-4-tertbutylbenzene; D) 1-butyl-4- methylbenzene.
Find out the structure of aromatic compound using the empirical formula and the products of chemical reactions: С9Н12, while chloration (kat. is present) only one monochlorderivative is created. Receive the compound from benzene. Write the equations.
Receive 2-chloro-5-nitrotoluene from benzene.
Write the equations and give the names of the products of mono- and dichloration of α-naphtol.
