
Lecture 6
Aromatic Hydrocarbons
Introduction
Nomenclature of the Derivatives of Benzene
The Stability of Benzene
Physical Properties of Aromatic Hydrocarbons
Preparation of Benzene
Reactions of Benzene
Polycyclic Aromatic Compound
Introduction
The term aromatic is used for historical reasons to refer the class of compounds related structurally to benzene
Benzene
Highly unsaturated
Six-membered ring compound with alternative single and double bonds between adjacent carbon atoms
Chemically unreactive compared to alkenes
2 Nomenclature of the Derivatives of Benzene
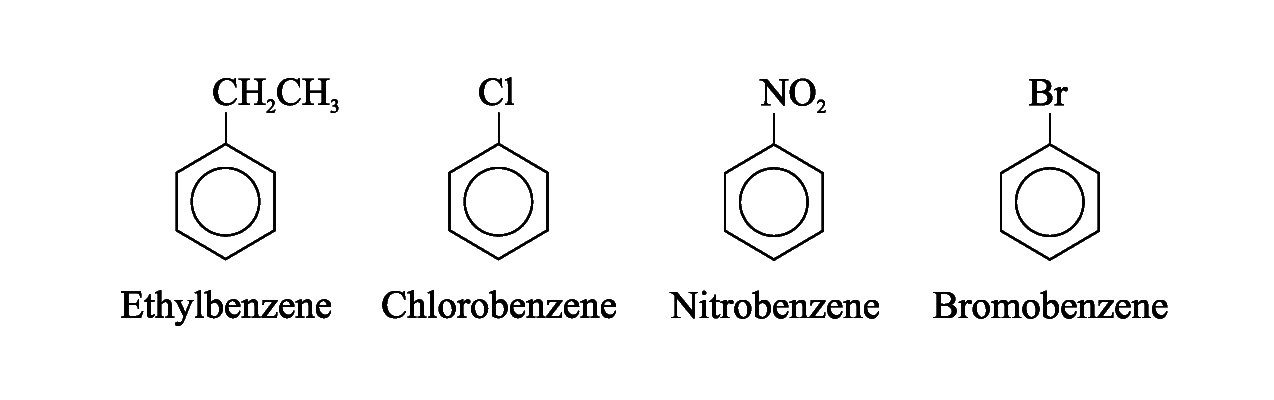
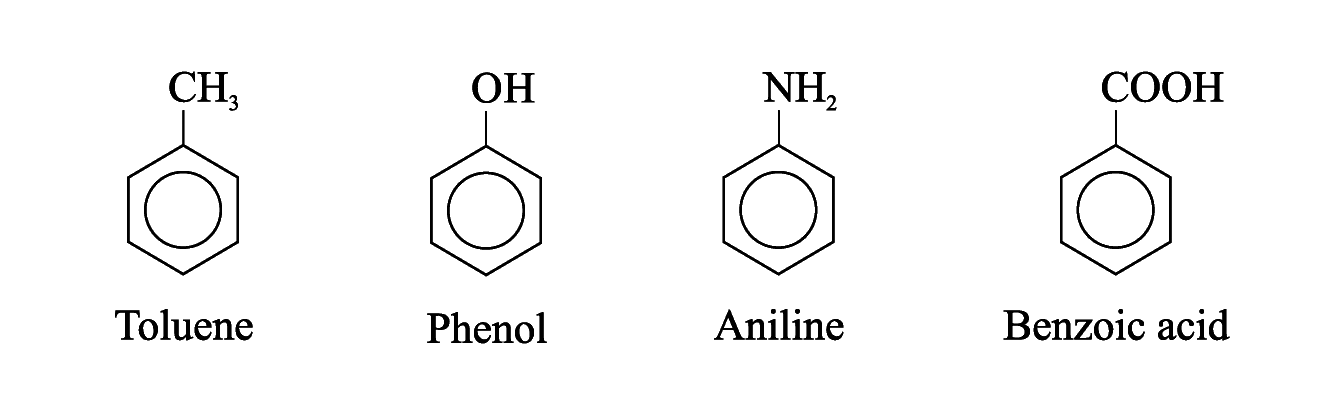
Monosubstituted benzenes
For certain compounds, benzene is the parent name and the substituent is simply indicated by a prefix
If the alkyl substitutent has more then six carbons, the compound is named as a phenil-substituted alkane.
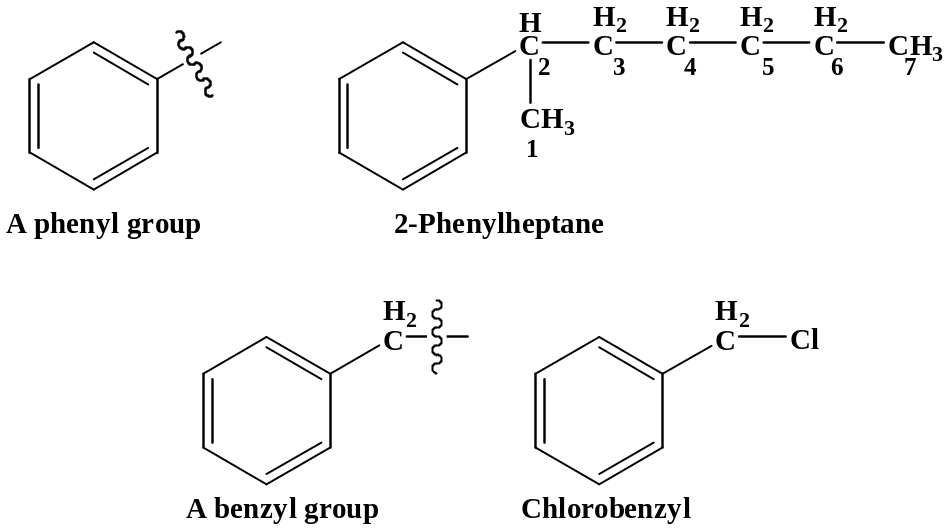
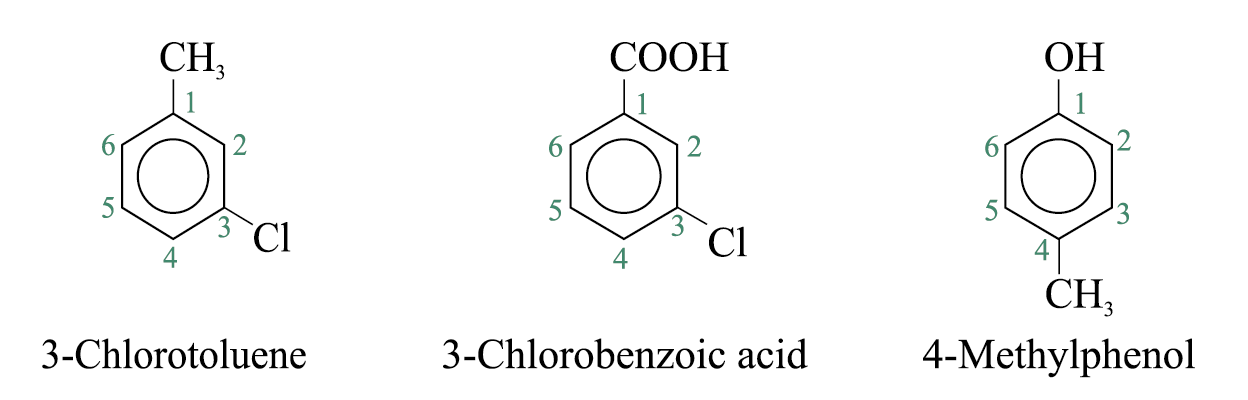
For other compounds, the substituent and the benzene ring taken together may form a new parent name
Polysubstituted benzenes
If more than one substituent are present and the substituents are identical, their relative positions are indicated by the use of numbers assigned on the ring. The prefixes ‘di-’, ‘tri-’, ‘tetra-’, and so on are used.
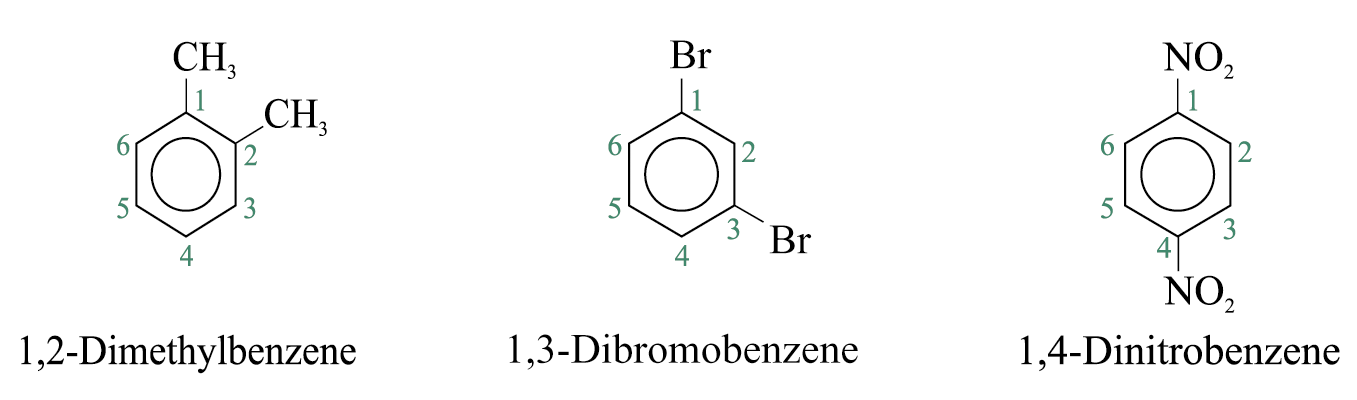
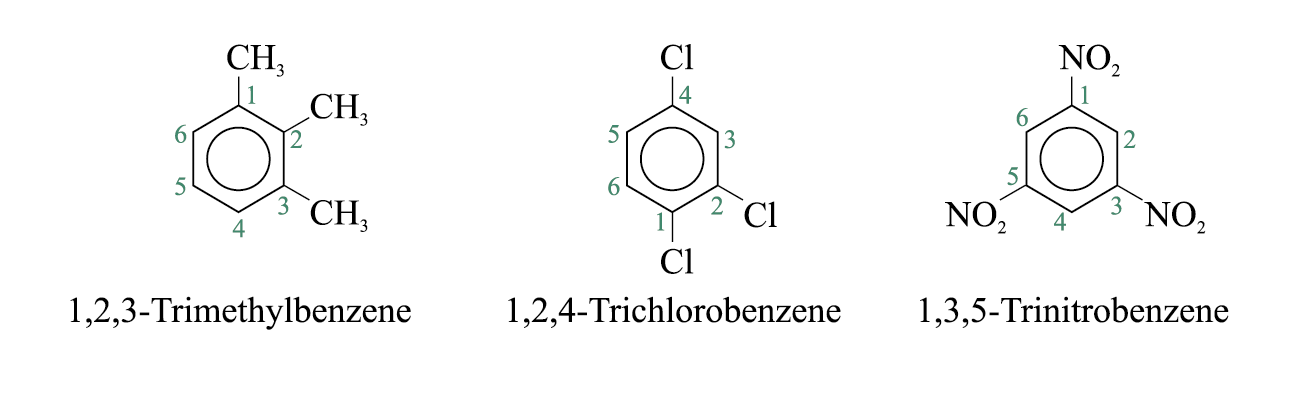
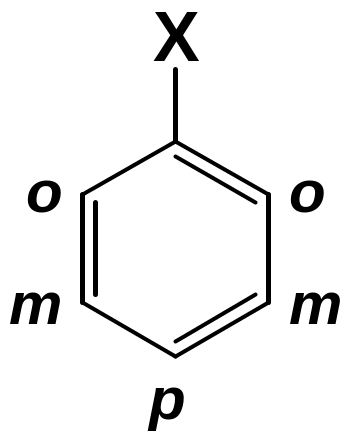
Disubstituted benzenes are named using one of the prefixes ortho (o) for 1,2 disubstituted, meta (m) for 1,3 disubstituted, para (p) for 1,4 disubstituted.
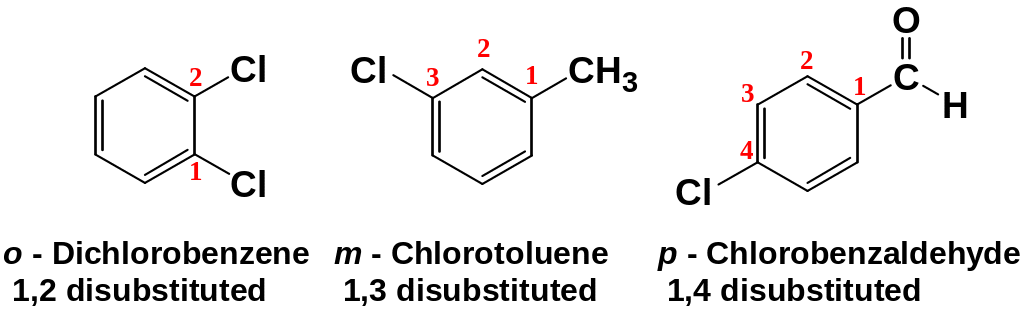
When more than one substituent are present and the substituents are different, they are listed in alphabetical order.
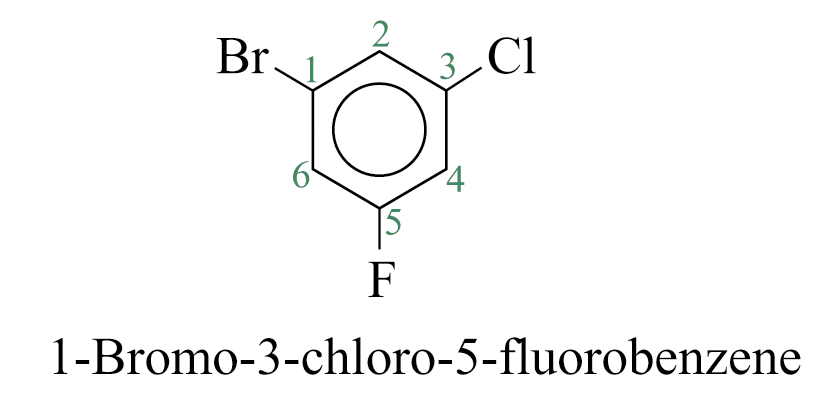
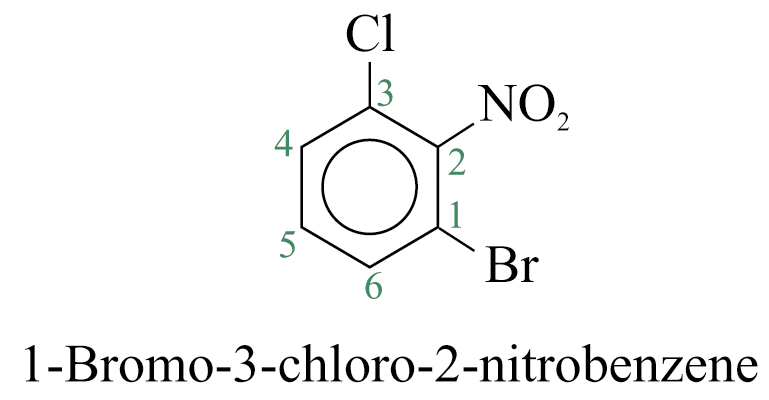
When a substituent is one that when taken together with the benzene ring gives a new parent name, that substituent is assumed to be in position 1 and the new parent name is used
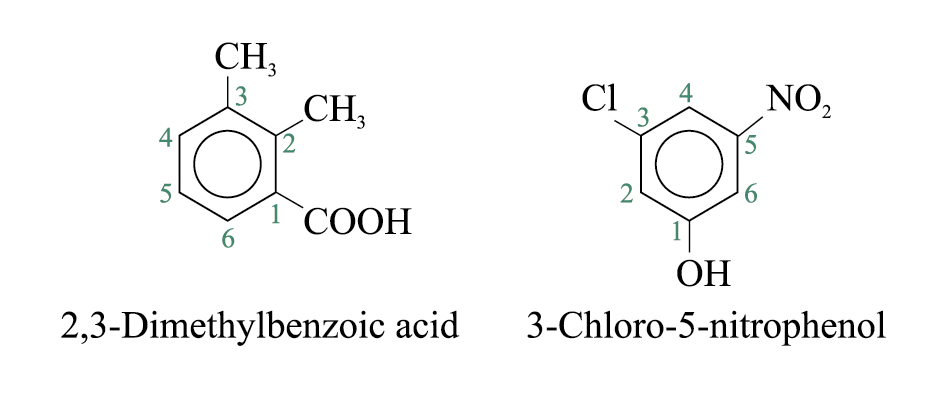
The Stability of Benzene
In 1865, Kekule proposed the structure of benzene:
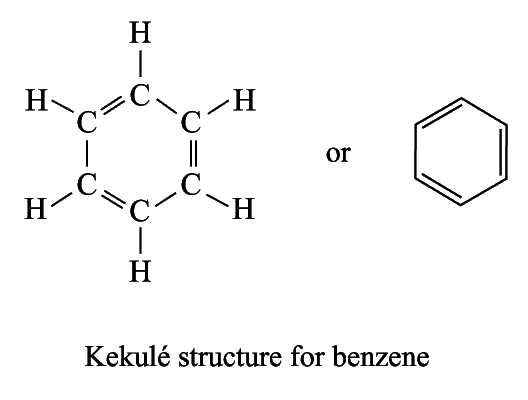
A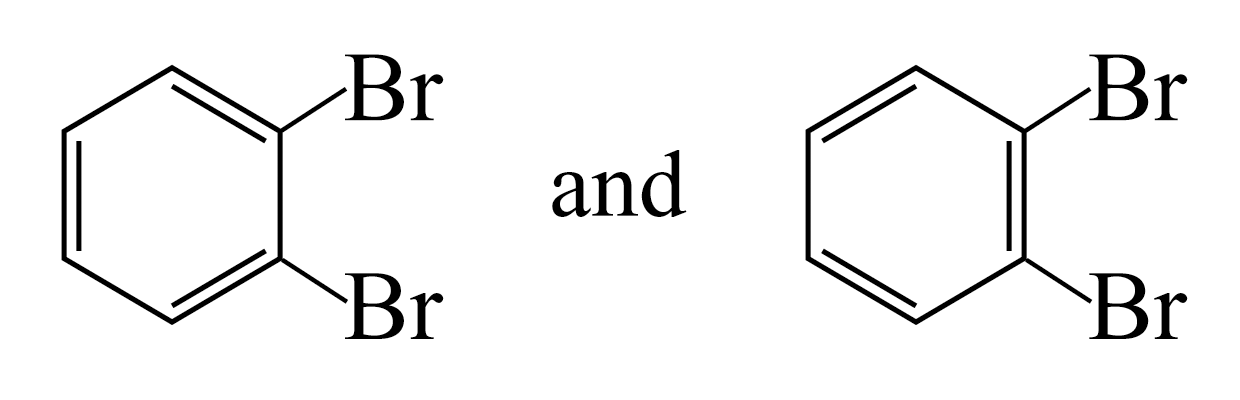 ccording
to the Kekulé structure, there should be two different
1,2-dibromobenzenes:
ccording
to the Kekulé structure, there should be two different
1,2-dibromobenzenes:
Only one 1,2-dibromobenzene has been found!!
According to the Kekulé structure, benzene should
undergo addition reactions readily
it gave substitution reaction products rather than addition reaction products
Þ Kekulé structure cannot explain the behaviour of benzene
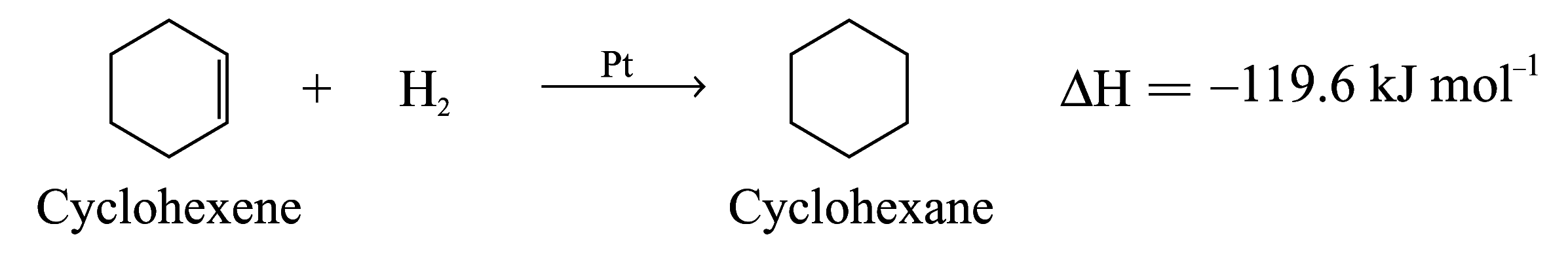
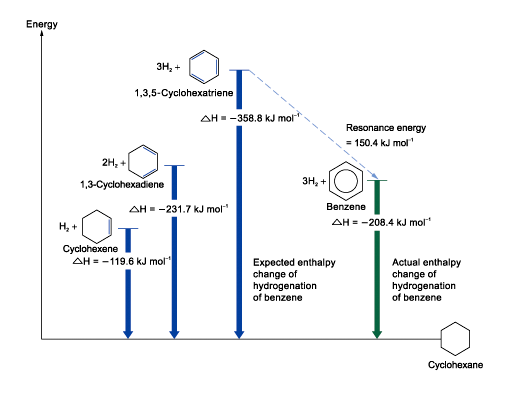
Enthalpy Changes of Hydrogenation of Benzene and Cyclohexene
Enthalpy change of hydrogenation of cyclohexene
= –119.6 kJ mol–1
The enthalpy change of hydrogenation of 1,3-cyclohexadiene is expected to be twice that of cyclohexene
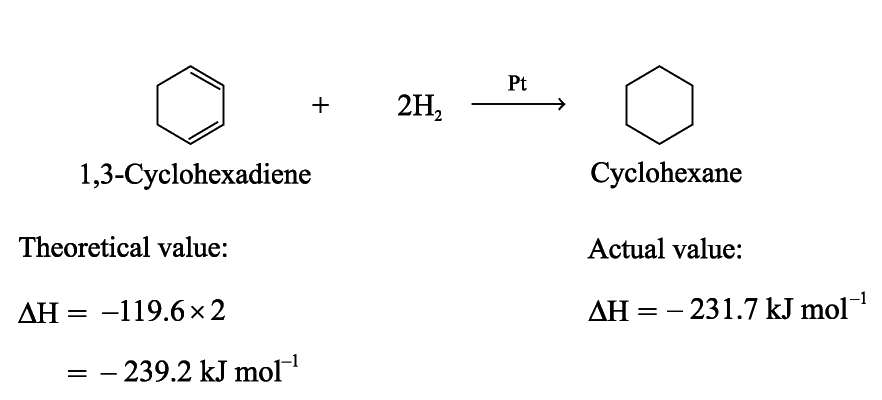
If benzene has the structure of 1,3,5-cyclohexatriene, The enthalpy change of hydrogenation is expected to be three times as much as that of cyclohexene
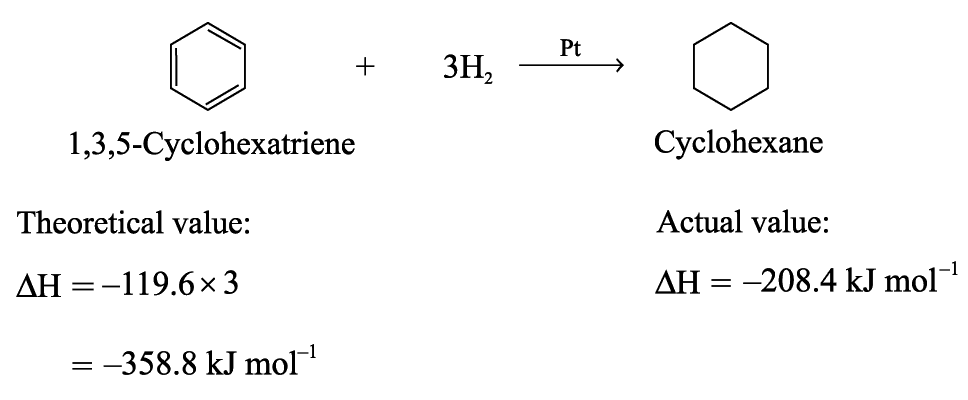
Benzene is more stable than Kekulé structure
The energy difference for the stabilization of benzene is called resonance energy of benzene
The Resonance Explanation of the Structure of Benzene
From X-ray crystallography,
The length of carbon-carbon bond in benzene is intermediate between C – C bond and C = C bond
0.134 nm > 0.139 nm > 0.154 nm C = C carbon bond in benzene C – C
All carbon atoms in benzene are sp2-hybridized
The side-way overlap of unhybridized 2p orbitals on both sides gives a delocalized p electron cloud above and below the plane of the ring
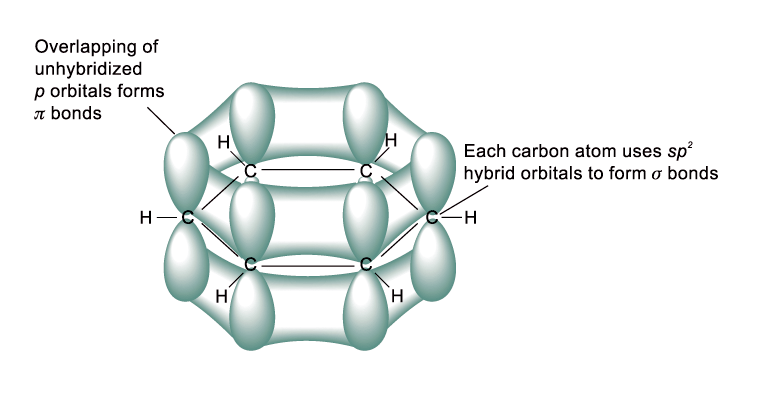
The delocalization of p electrons gives benzene extra stability and determines the chemical properties of benzene
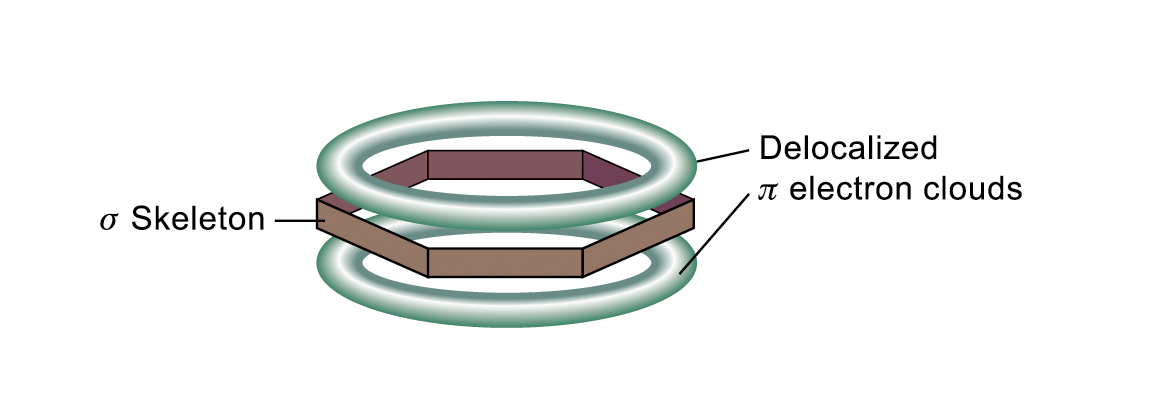
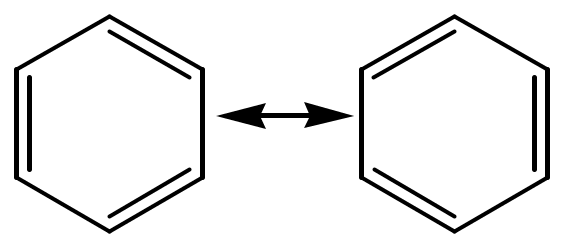
Benzene is described by resonance theory as a resonance hybrid of two equivalent Kekulé structures:
S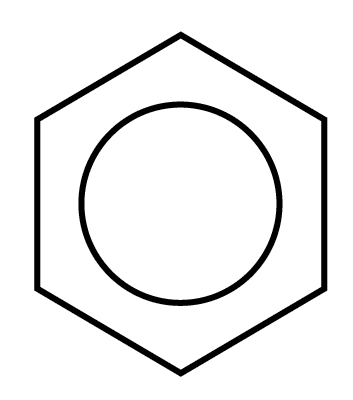 tructural
formula of benzene:
tructural
formula of benzene:
The circle represents the six electrons that are delocalized about the six carbon atoms of the benzene ring
Benzene is an aromatic compound.
Benzene is described by molecular orbital theory as a planar, cyclic, conjugated with six π electrons.
Hückel rule:
A molecule must be planar, cyclic, conjugated and have 4n+2 π electrons (where n = 0, 1, 2, 3, and so on) to be aromatic.
Planar, cyclic, conjugated molecule with other number of π electrons are antiaromatic.
Alkylbenzenes are a group of aromatic hydrocarbons in which an alkyl group is bonded directly to a benzene ring
also known as arenes
e.g.
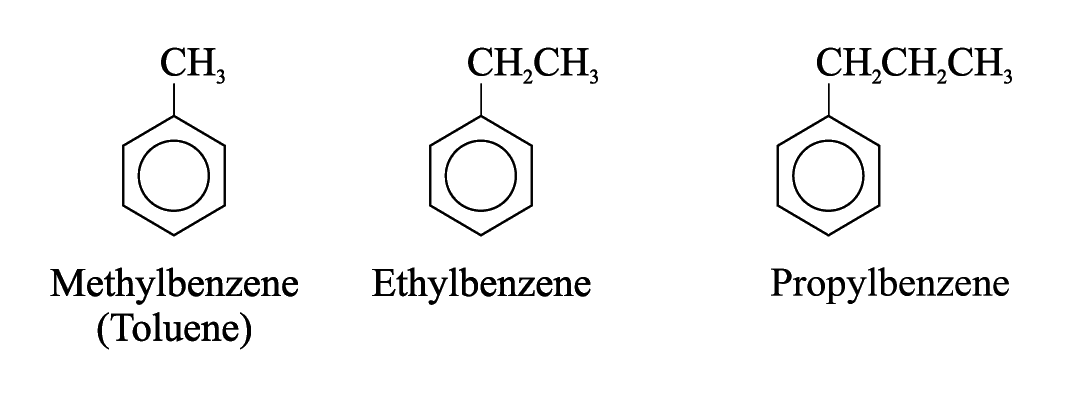
S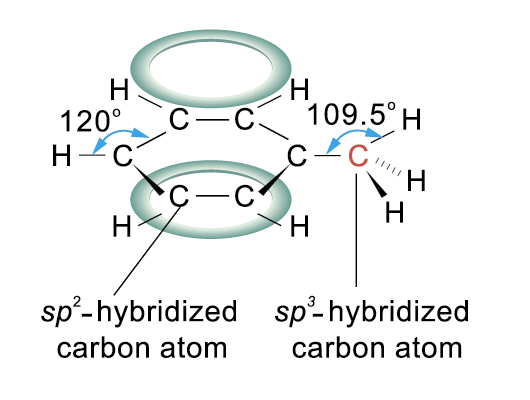 tructure
of Methylbenzene
tructure
of Methylbenzene
All C atoms in the ring is sp2-hybridized
The C atom in the methyl group is sp3-hybridized
The delocalized p electron clouds give rise to extra stability
