3 Nomenclature of the Derivatives of Hydrocarbons
Nomenclature of Compounds with One Type of Functional Group
Compounds with functional groups that must be designated as prefixes

1. The carbon chains are named and numbered in the usual way. Numbers are assigned to functional groups in the same way as the alkyl substituents. e.g.
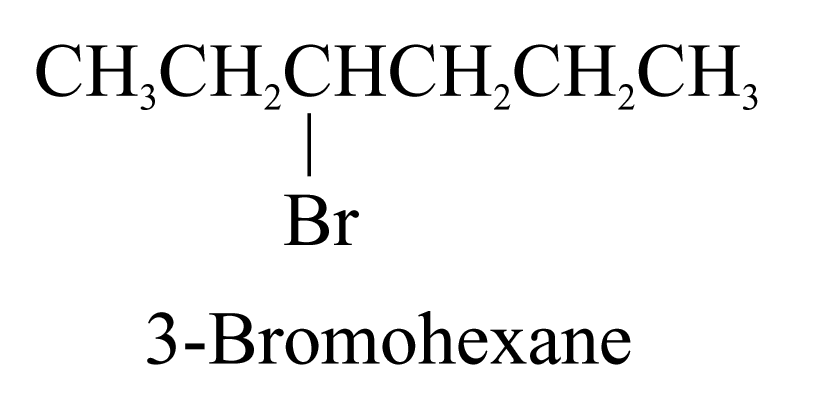
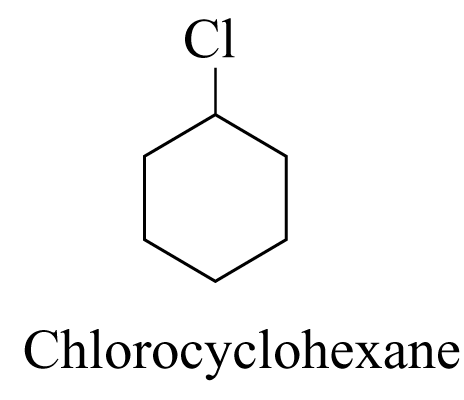
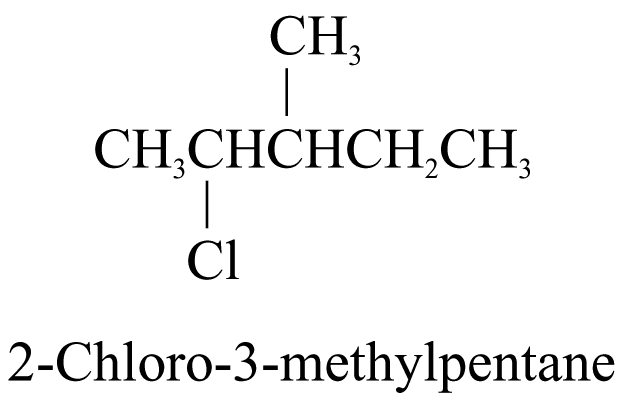
2. When the parent chain has both alkyl groups and other substituents, the chain is numbered from the end nearer the first substituent, regardless of what substituents are. All the prefixes are then arranged in alphabetical order. e.g.
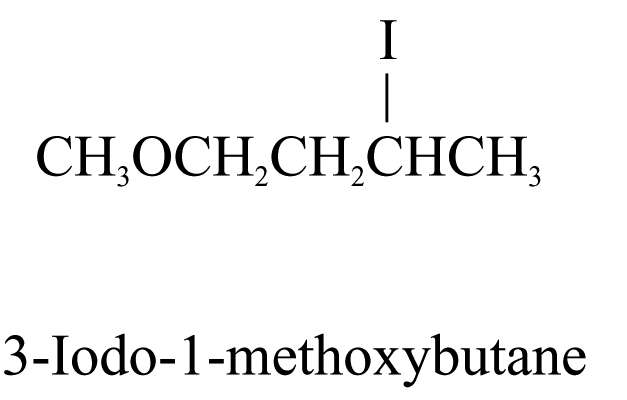
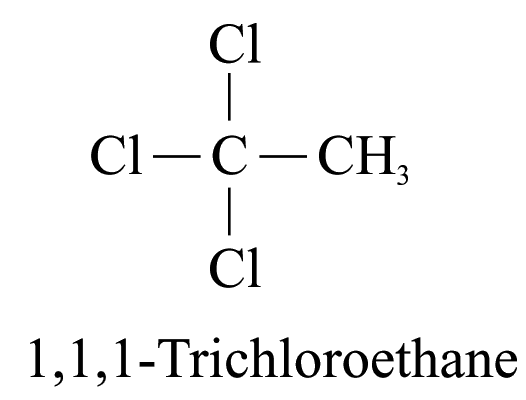
3. When two or more substituents are identical, indicate this by the use of the prefixes ‘di-’, ‘tri-’, ‘tetra-’, and so on. e.g.
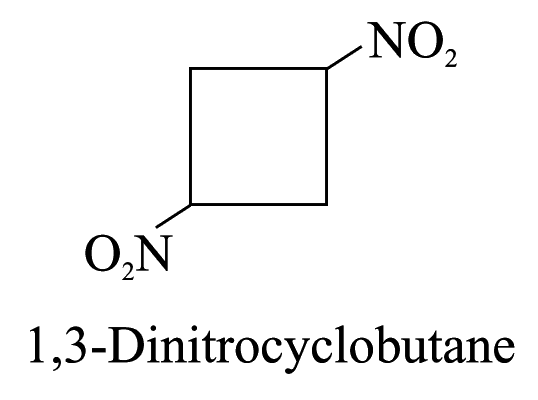

Compounds with functional groups that may be designated as prefixes or suffixes



Compounds with functional groups that may be designated as prefixes or suffixes

Principal functional group is the functional group expressed as a suffix and has priority over unsaturated centres.
Parent carbon chain is chosen to include the longest possible carbon chain and maximum number of principal functional groups.
The carbon chain is numbered to give the principal functional group the lower number.
The position of the principal functional group is indicated by using this number, and the positions of other substituents are indicated by using the numbers corresponding to their positions along the parent carbon chain.
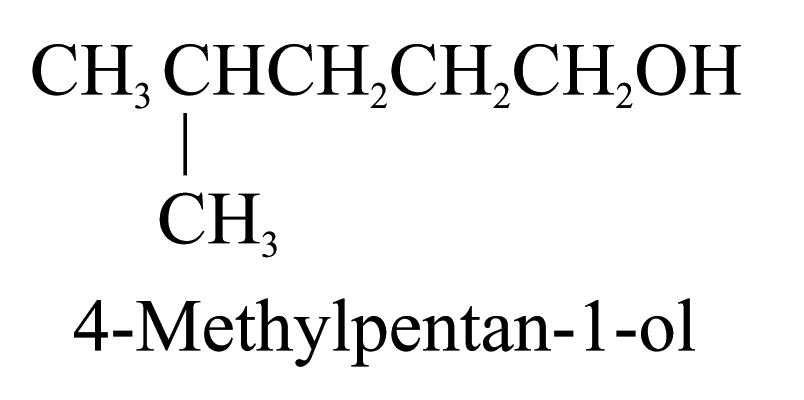
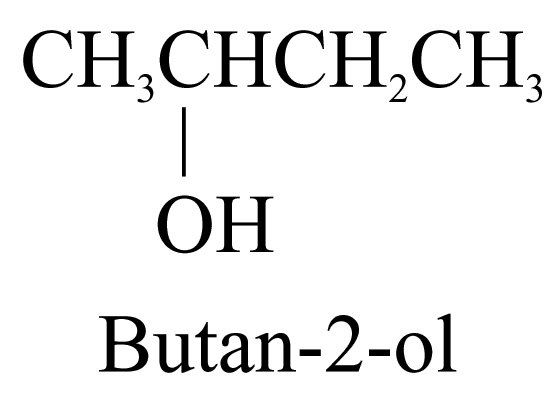
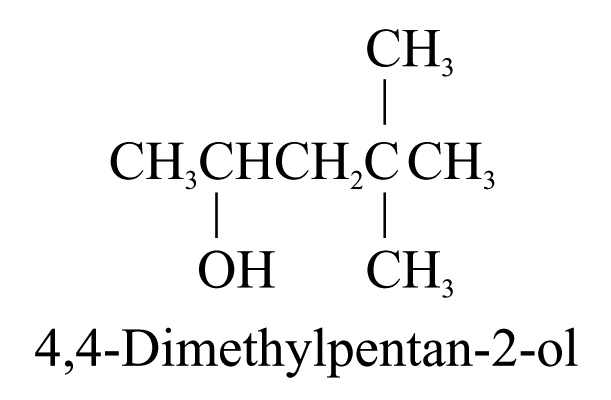
1. Alcohols (ending ‘-ol’)
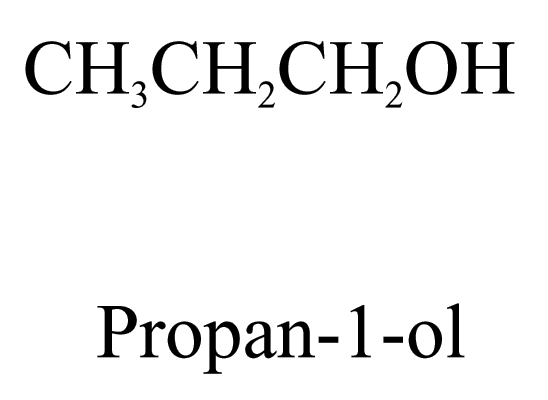

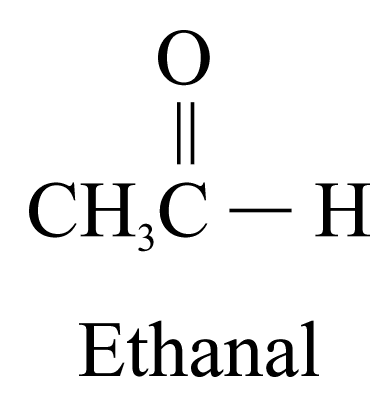
2. Aldehydes (ending ‘-al’) and ketones (ending ‘-one’)
The carbon
atom of the carbonyl group
![]() is
included in the parent carbon chain.
is
included in the parent carbon chain.
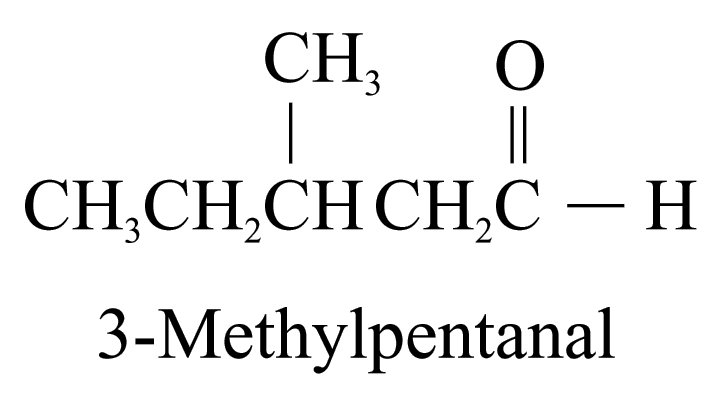
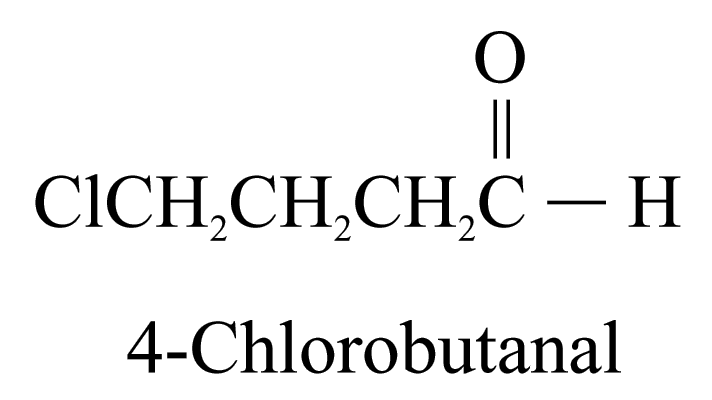
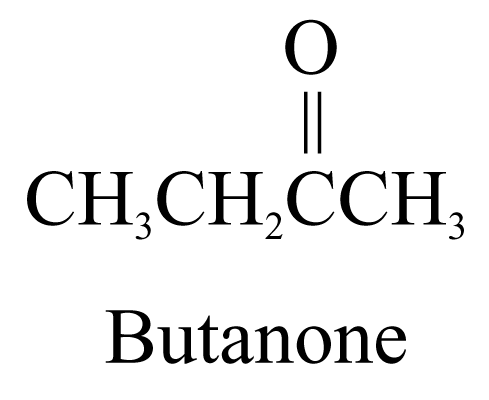
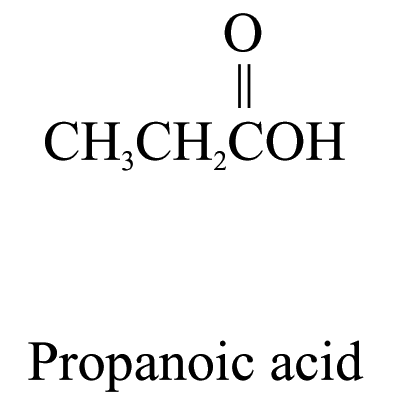
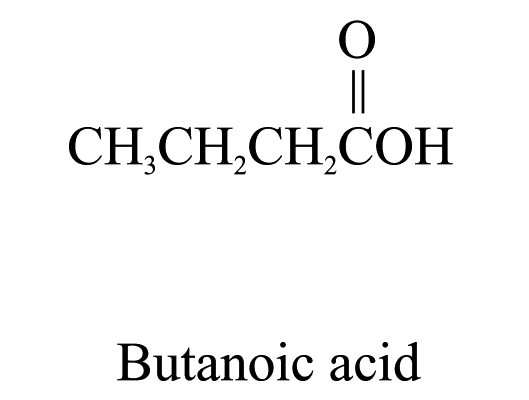
3. Carboxylic acids (ending ‘-oic acid’)
The carbon atom of the carboxyl group (i.e.![]() )
is included in the parent carbon chain.
)
is included in the parent carbon chain.
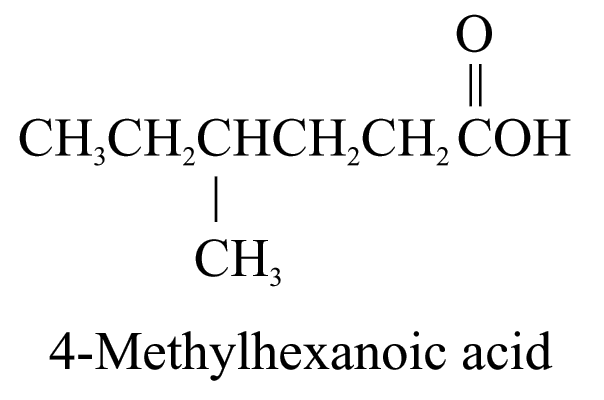
4. Acyl chlorides (ending ‘-yl chloride’)
The carbon
atom of the
![]() group
is included in the parent carbon chain.
group
is included in the parent carbon chain.
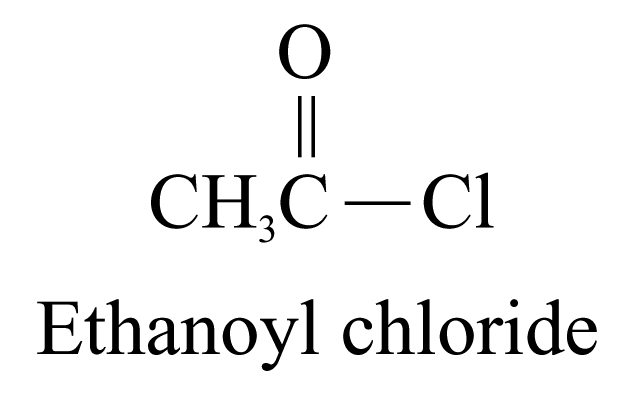
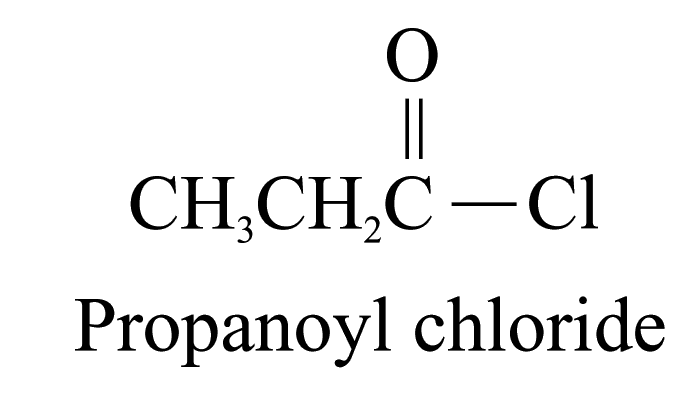
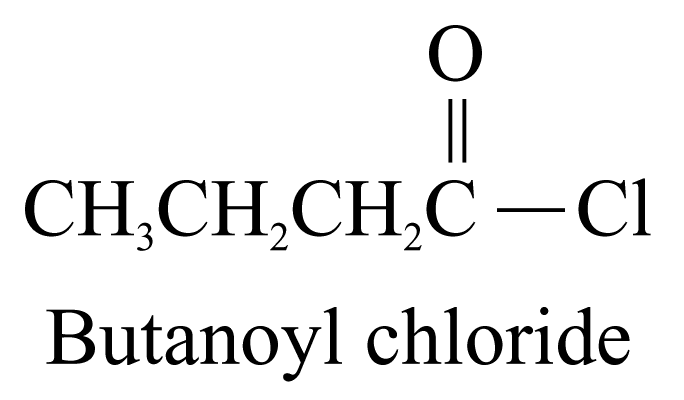
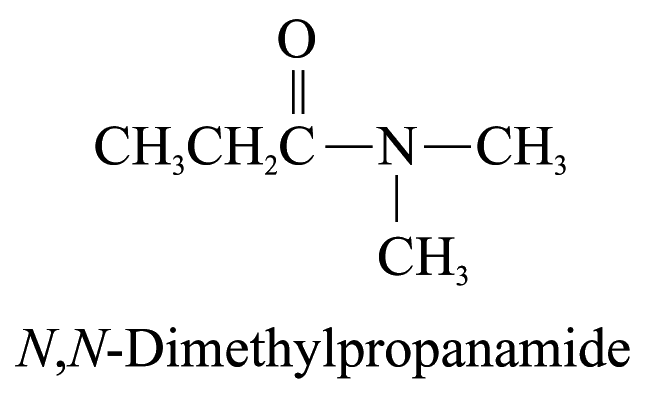
5. Amides (ending ‘-amide’)
The carbon
atom of the amide group (i.e.
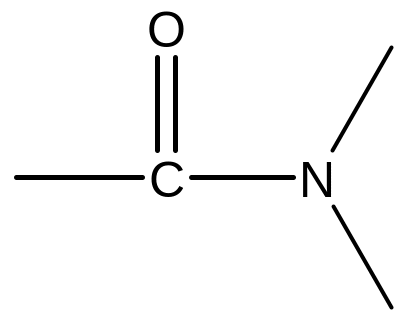 )
is included in the parent carbon chain. Alkyl groups on the nitrogen
atom of amides are named as substituents and the named substituent
is preceded by N-
or N,N-.
)
is included in the parent carbon chain. Alkyl groups on the nitrogen
atom of amides are named as substituents and the named substituent
is preceded by N-
or N,N-.
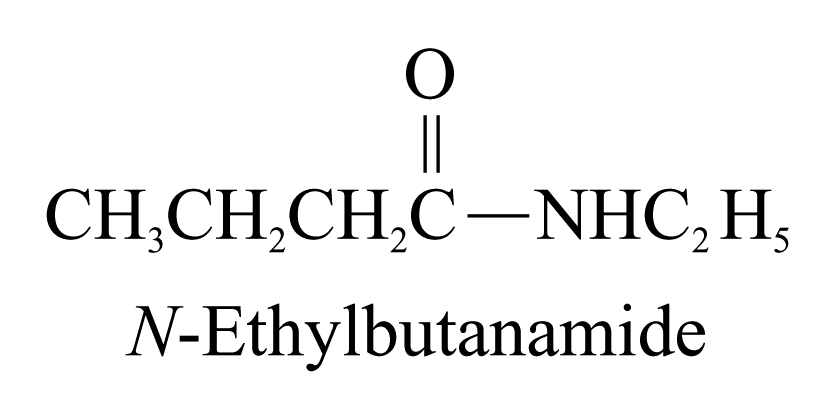
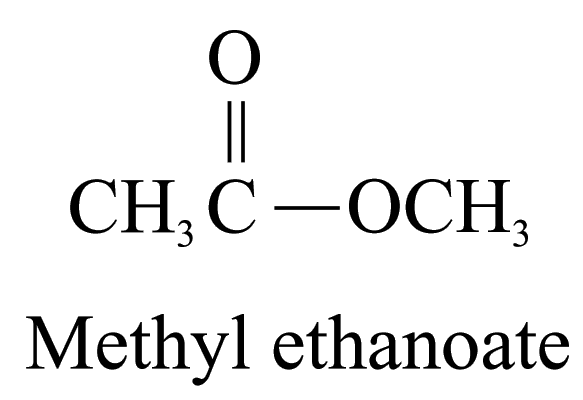
6. Ester (ending ‘-oate’)
The name of ester is derived from the names of the alcohol (with the ending ‘-yl’) and the carboxylic acid (with the ending ‘-oate’) forming the ester. The portion of the name derived from the alcohol comes first, and then the carboxylic acid.
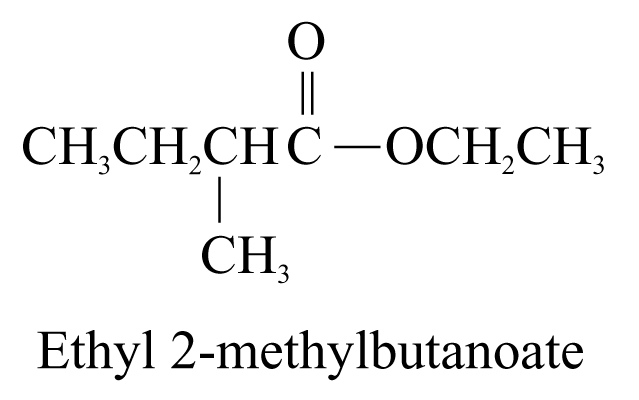
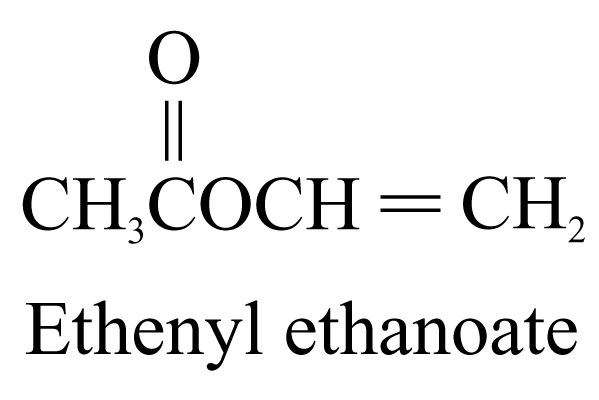
Nomenclature of Compounds with More than One Type of Functional Group
1. Only one of the functional groups can be designated as the ending of the name. This is the principal functional group.
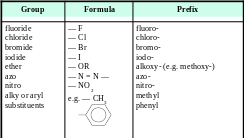
The priority of choosing principal functional group in decreasing order is listed in the following table.
The group that is highest in the list precedes all other groups and become the principal functional group in the compound to be named.
Group |
Formula |
Prefix |
Suffix |
Carboxylic acid |
|
carboxy |
-oic acid |
Sulphonic acid |
|
sulpho |
-sulphonic acid |
Ester |
|
— |
-oate |
Acyl halide |
|
— |
-oyl halide |
Amide |
|
— |
-amide |
Nitrile |
|
cyano |
-nitrile |
Aldehyde |
|
oxo |
-al |
Ketone |
|
oxo |
-one |
Alcohol |
|
hydroxy |
-ol |
Amine |
|
amino |
-amine |
2. The principal functional group has priority in the selection of the longest possible carbon chain and the choice of lowest number. Other groups are designated as prefixes and listed in alphabetical order.
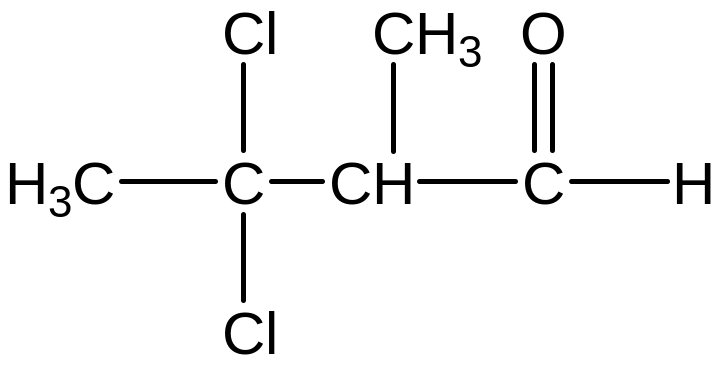
Example
1: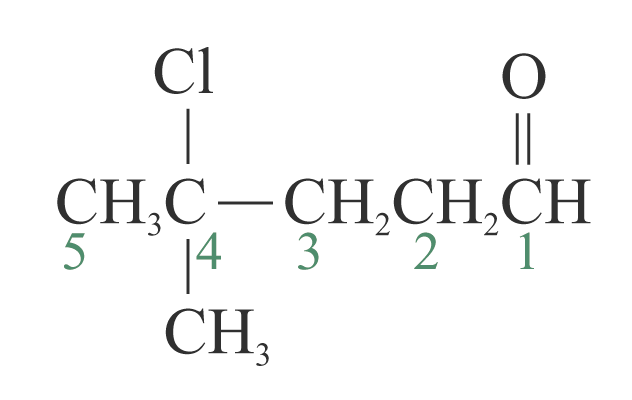 4-chloro-4-methylpentanal
Example 2:
4-chloro-4-methylpentanal
Example 2: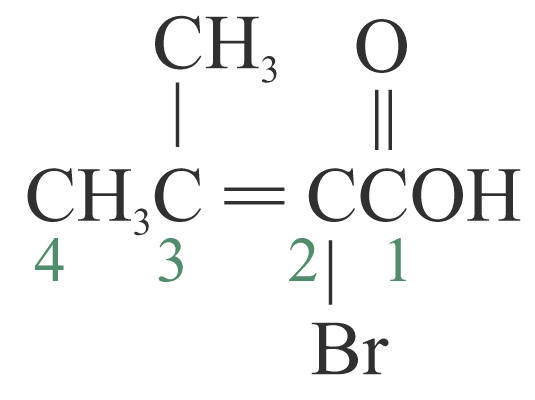 2-bromo-3-methylbut-2-enoic
acid
2-bromo-3-methylbut-2-enoic
acid
Example
3: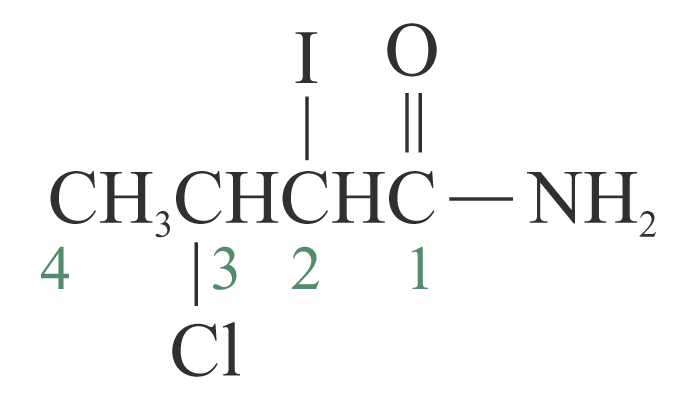 3-chloro-2-iodobutanamide
3-chloro-2-iodobutanamide
Example
4:
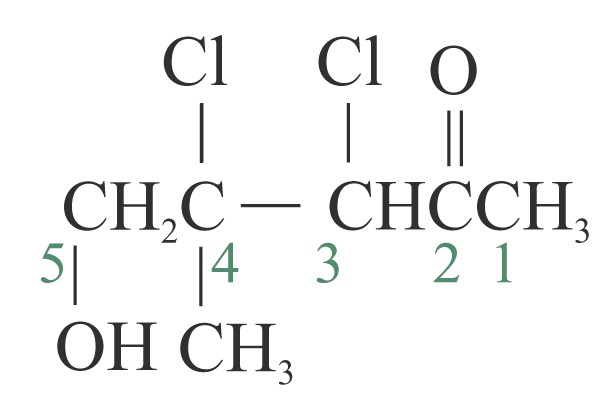 3,4-dichloro-5-hydroxy-4-methylpentan-2-one
3,4-dichloro-5-hydroxy-4-methylpentan-2-one
Example
5:
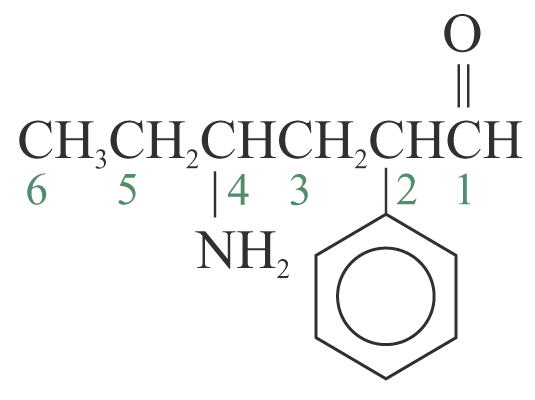 4-amino-2-phenylhexanal
4-amino-2-phenylhexanal
Monosubstituted Aromatic Hydrocarbons
1. Some monosubstituted aromatic hydrocarbons can be named by adding the name of the substituents as prefixes to the name of the aromatic hydrocarbon. e.g.
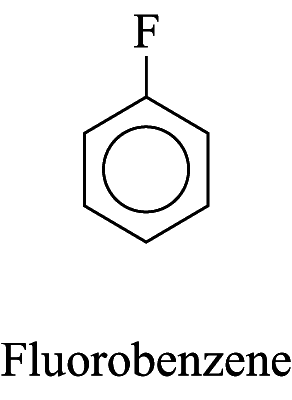
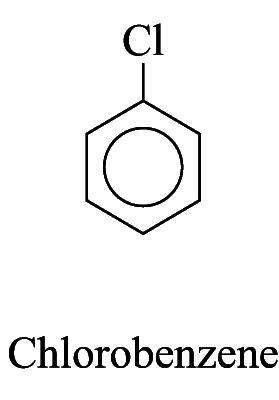
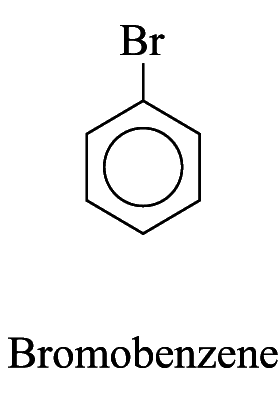
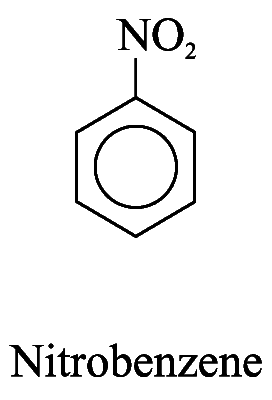
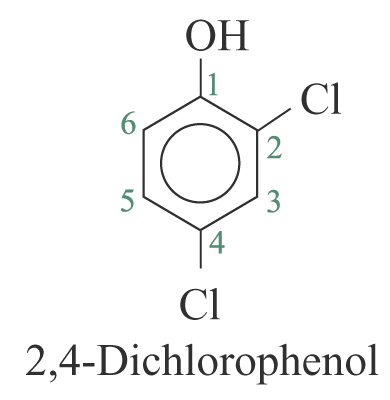
2. For other monosubstituted aromatic hydrocarbons, the substituent and the benzene ring taken together may form a new parent name. e.g.
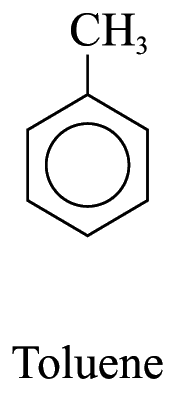
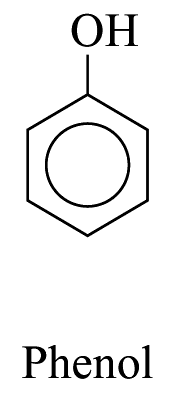
![]()
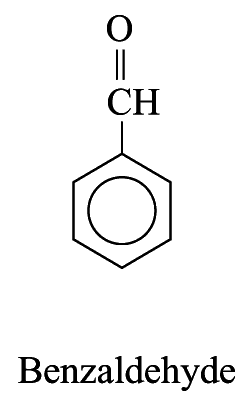
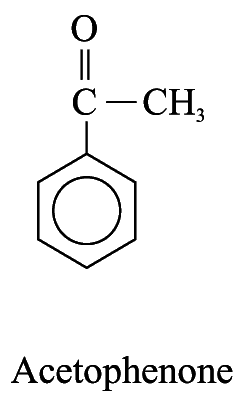
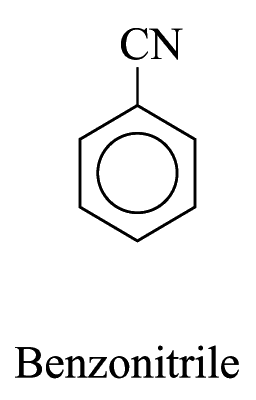
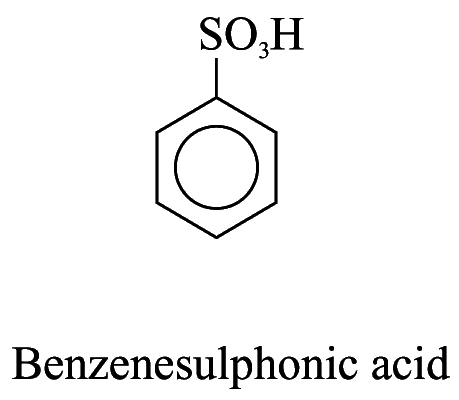
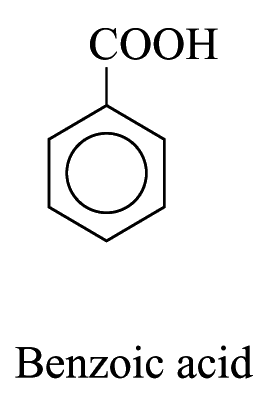
Polysubstituted Aromatic Hydrocarbons
When two or more substituents are identical, indicate this by the use of the prefixes ‘di-’, ‘tri-’, ‘tetra-’ and so on. The benzene ring is numbered so as to give the lowest possible numbers to the substituents.
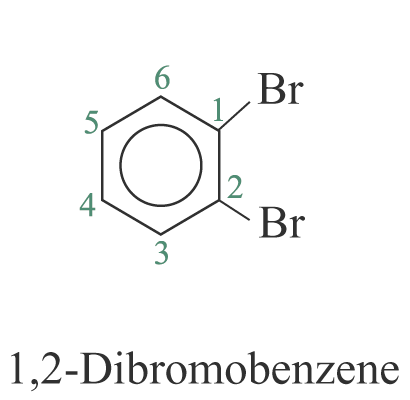
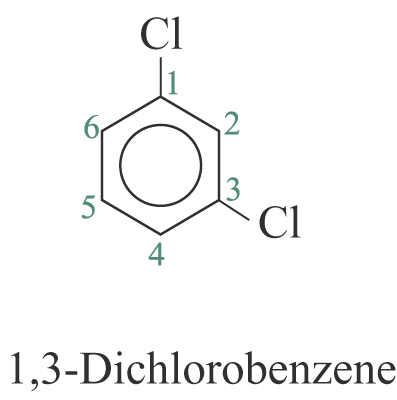
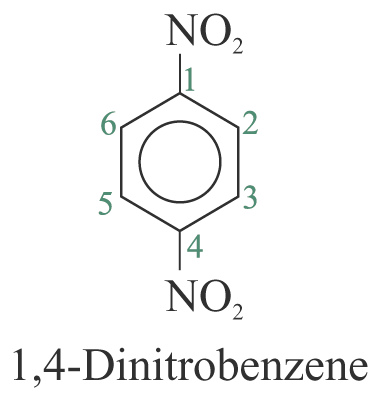
2. When the substituents are different, they are listed in alphabetical order. e.g.
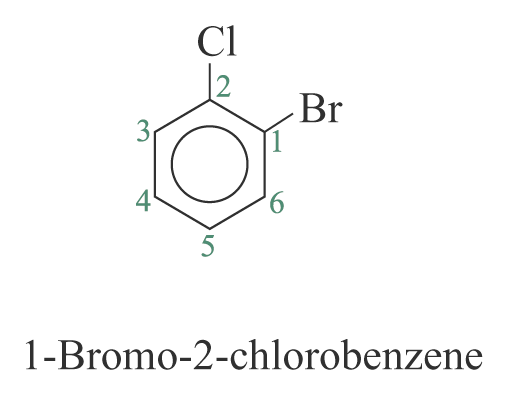
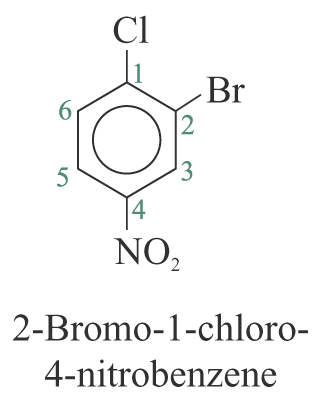
3. When a substituent is one that when taken together with the benzene ring to give a new parent name, that substituent is assumed to be in position 1 and the new parent name is used. e.g.
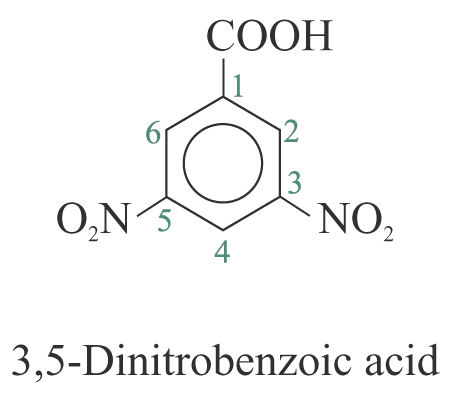
Questions and problems
Write the structural formulas of the following hydrocarbons and radicals:
2,3,5-trimethylhexane; 1.2-dimethylpropyl.
 Назвіть
за систематичною номенклатурою наведену
сполуку. Скільки первинних, вторинних,
третинних та четвертинних вуглецевих
атомів містить ця сполука?
Назвіть
за систематичною номенклатурою наведену
сполуку. Скільки первинних, вторинних,
третинних та четвертинних вуглецевих
атомів містить ця сполука?
а) I–6, II-3, III-2, IV-1; б) I–7, II-1, III-5, IV-0; в) I–6, II-3, III-2, IV-1; г) I–7, II-3, III-1, IV-2.
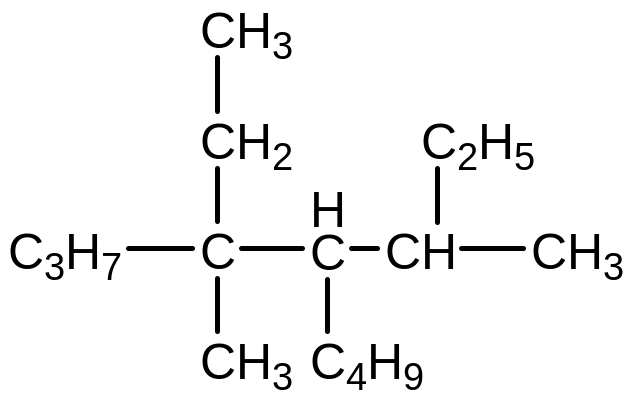
Write the structural formula of disecbutylethylmethane and give its name using the IUPAC nomenclature
Give the name to the following compound using the IUPAC nomenclature: