
Lecture 1
General Organic Chemistry (1)
1 Introduction to organic chemistry
Structure of organic compounds
Methods of representation of organic molecules
A cross-coupling of atoms in a molecule
Acids and Bases
Introduction to organic chemistry
Organic Chemistry: The chemistry of the compounds of carbon
Organic chemistry is the study of compounds containing carbon. It is called «organic" because scientists used to think that these compounds were found only in living things or fossils. However, vast numbers of different carbon-containing compounds can now be produced artificially in laboratories and factories, for use in industry. For example, drugs, plastics, and pesticides are all synthetic organic substances. About 5 millions of the 5,5 millions compounds known today contain carbon.
Carbon compounds: DNA, proteins in our body, carbohydrates, wool, cotton, petrol, medicines, pesticides, etc. Organic chemicals make our life easy but also cause serious environmental problems (e.g. organic compounds used as aerosol propellent damage the ozone layer, insecticide is harmful to human)
In 1828, Wohler (a German chemist)

(Inorganic compound) (Organic compound)
Redefining …...
Organic chemistry is the study of carbon compounds except carbon monoxide, carbon dioxide, carbonates, hydrogencarbonates, carbides and cyanide.
2. Structure of organic compounds
The theory of the chemical structure OF ORGANIC COMPOUNDS
A.M. Butlerov is the Great Russian scientific plays a decided role in making a theory of the chemical structure of organic compounds. In 1861, 19 September on the 36-th convention of German naturalists Butlerov has promulgated his theory in the report «On the chemical structure of substance».
The main positions of Butlerov’s theory of the chemical structure are the following.
All atoms in the molecule of organic compound are bound with each other in determined sequences in accordance with their valence. Changing a sequence of atoms location brings about forming a new substance with new properties. For instance, the substance С2Н6О can exist as two different compounds: dimethyl ester (СН3-О-СН3) and ethyl alcohol (С2Н5ОН).
Properties of substance depend on the chemical structure. Chemical structure - is a certain order in the alternating of atoms in the molecule, in the interaction and mutual influence of atoms a to each other - as nearby, so and through other atoms. As a result each substance has their own particular physical and chemical properties. For example, dimethyl ester - is a gas with no smell, insoluble in water, melting point (-138°C), boiling point (23.6°C); ethyl alcohol- is a liquid with the smell, soluble in water, melting point (-114.5°C), boiling point (78.3°C). Given position of the theory of the chemical structure has explained a phenomenon of isomerism widespread in the organic chemistry. Figured pair compounds- dimethyl ester and ethyl alcohol- is the one of the examples illustrating a phenomenon of isomerism.
Study of substances properties allows defining their chemical structure, but the chemical structure of substances defines their physical and chemical properties.
Carbon atoms are capable to be connected between itself, forming carbon with different type chains. They can be as opened, so and closed (round-robin), as direct, so and ramified. Chains can be saturated (with single bonds) or unsaturated (with double and triple bonds), it depend on the numbers of bonds consumed by carbon atoms on the united with each other.
Each organic compound has one determined structured formula, which builds, basing on the position about four-valence carbon and on the ability of its atoms to form chains and cycles. Molecule structure as a real object is possible to study by means of chemical and physical experimental methods.
A.M. Butlerov has not only substantiated the theory of the chemical structure of organic compounds he has also carried out a number of experiments, having confirmed predictions of his theory by the reception of isobutane, butyl alcohol and etc. These facts enable A M. Butlerov to declare in 1864 that available facts allow vouching for the possibility of synthesis getting any organic material.
In the sequel development and motivation of the theory of the chemical structure of organic compounds Butlerov followers (V.V. Markovnikov, E.E. Vagner, N.D. Zelinsky, A.N. Nesmeianov) has played a great role.
The structure of atom on the quantum-mechanical point.
This point of view is based on the solution of wave equation of Shredenger, that is reduced to determination of possibility оf location of electron that has energy Е in some point of space in some moment of time.
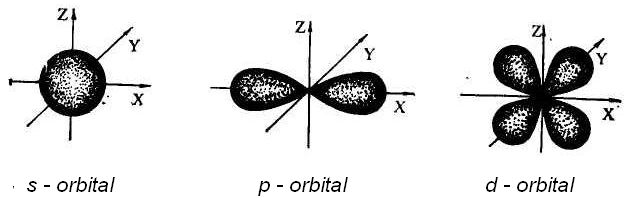
Orbital is the part of space, in which the probability of electron location is about 95%. There are s, p, d, f – atomic orbitals, that has different energy. Atomic orbital, which doesn’t consist electrons, is called vacant.
The solution of the equation of Shredenger needs the introducing of four quantum numbers for the full characteristic of electron:
п – the main quantium number, that gives the characteristic to the distance from the electron to the nucleus, and to electron’s energy (from 1 to ∞). Every п corresponds with the energy level.
l – orbital quantum number, that determines the form of electron orbital (from 0 to п-1).
m – magnetic quantum number, that shows the location of electron orbitals in space (from –l to +l) and the orientation of every one comperatively to another one.
s – spin quantum number that gives the characteristic to the rotation of electron around it’s own axis (-1/2; +1/2).
Electron configuration of atom is determined by the following rules:
(Hund's rule ) For orbitals with the same energy, the lowest energy is attained when the number of electrons with the same spin is maximized.
(Pauli principle) No two electrons in atom can have the same set of four quantum numbers.
(Klechkovsky’s rule) Every new orbital is filled only after orbitals with lower energy are filled.
Types of Chemical Bonds
Covalent
Ionic
Donor-acceptor
Hydrogen
Ability to Form Multiple Bonds
Carbon atoms are able to form single, double and triple bonds.
E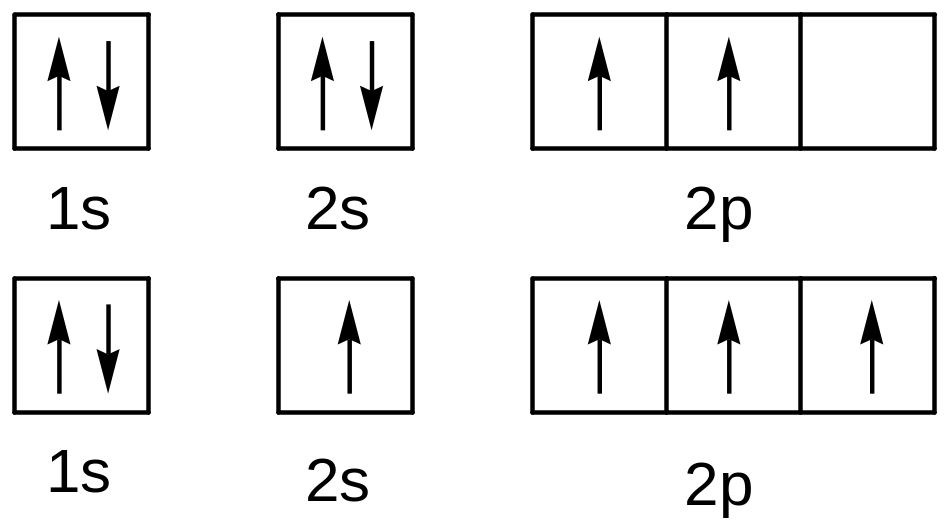 lectronic
configuration of carbon (ground state) : 1s22s22p2
lectronic
configuration of carbon (ground state) : 1s22s22p2
Each carbon atom has four unpaired electrons when excited and tend to form 4 covalent bonds.
s p3
Hybridization
p3
Hybridization

М еthane
(СH4)
еthane
(СH4)
σ-bonding – the bonding that is created by the overlap of s-, p- or hybrid orbitals along the axis that unites the nuclei of two atoms. This bonding permits the rotation of atoms around the bond.
Hybrid orbitals forms σ-bonds only.
![]()
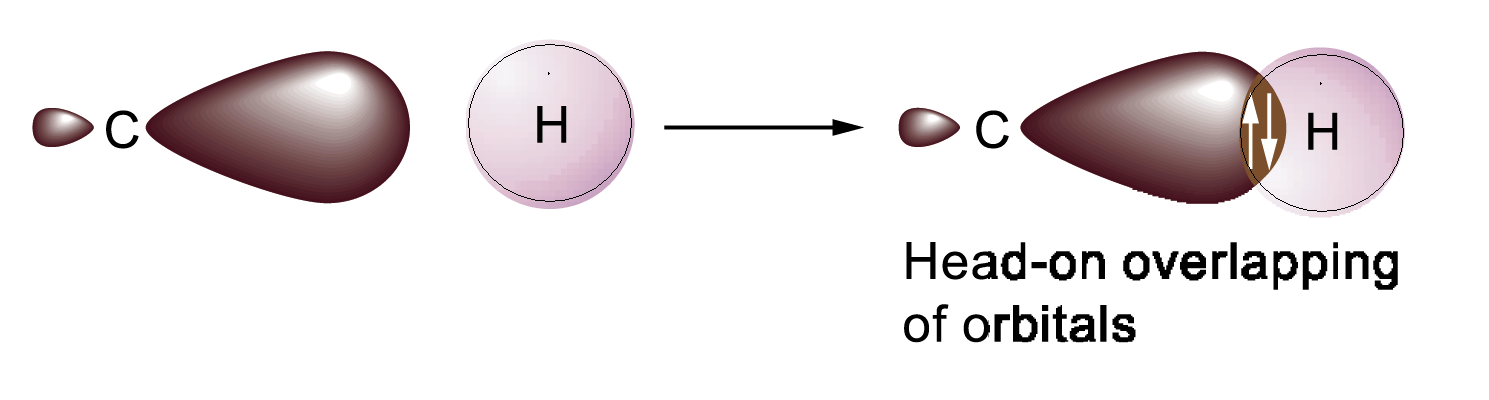
sp3 hydrid orbital
E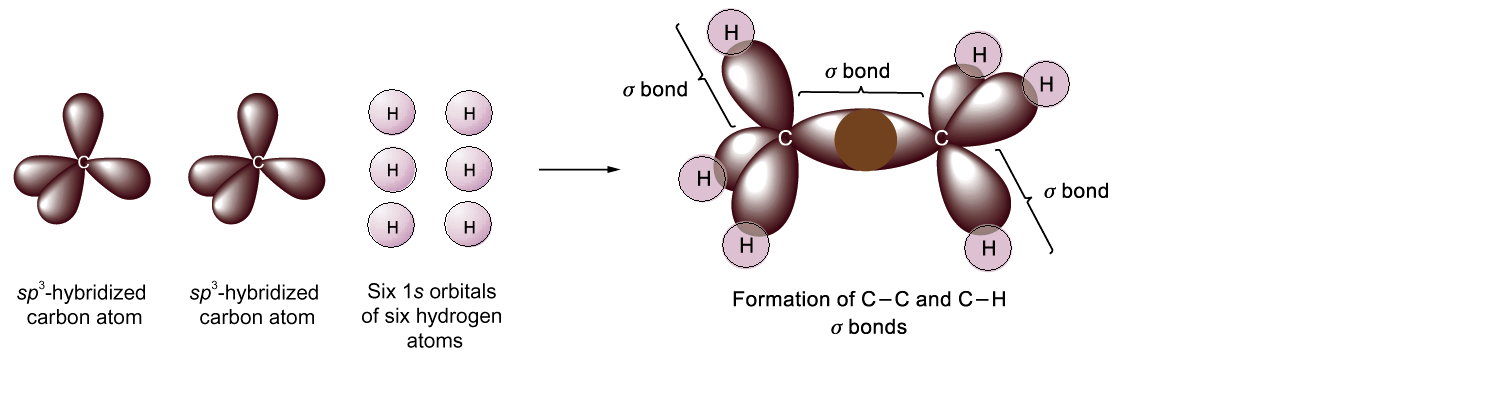 thane
(С2H6)
thane
(С2H6)
Electron Pair Repulsion Theory predict the geometry of arrangement of atoms in the molecules
Electron pairs tend to stay as far apart as possible (electronic repulsion between lone pairs is generally greater than that between bond pairs)
e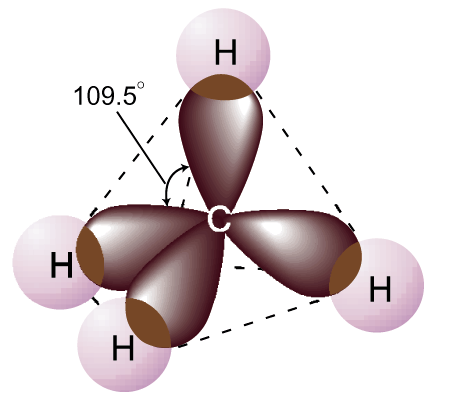 .g.
Methane has tetrahedral orientation electron pairs to have the
maximum separation of 109.5°
.g.
Methane has tetrahedral orientation electron pairs to have the
maximum separation of 109.5°
