
Lecture 3
General Organic Chemistry (3)
Classification of Organic Compounds
Nomenclature of Hydrocarbons
Nomenclature of the Derivatives of Hydrocarbons
Nomenclature of the Derivatives of Aromatic Hydrocarbons
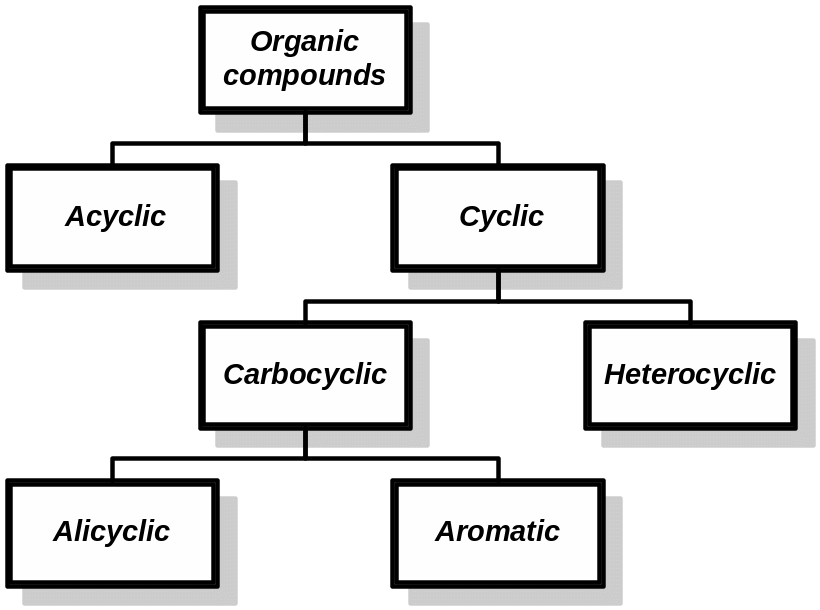
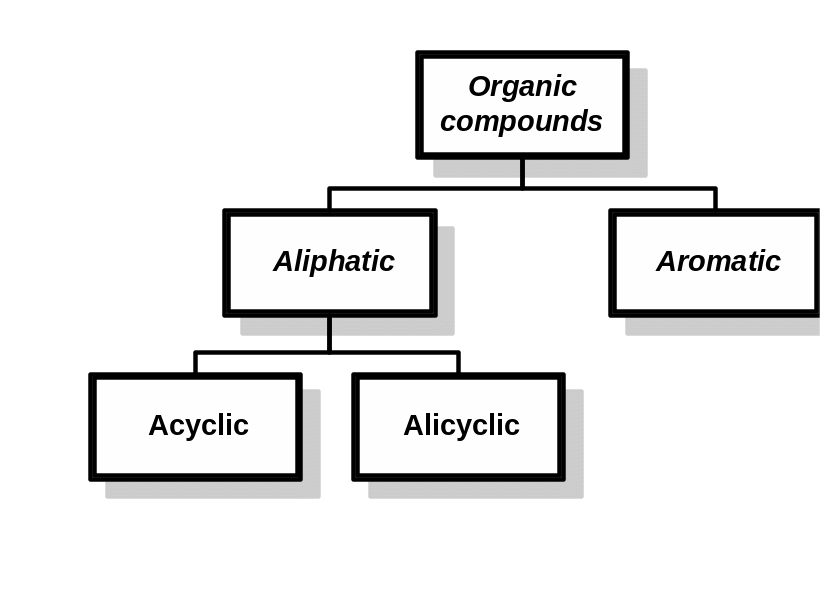
1 Classification of Organic Compounds
Classification by carbon skeletons
Classification by Functional Groups
The compounds in a particular family are characterized by the presence of a certain arrangement of atoms called a functional group
A functional group is defined as an atom or a group of atoms that effectively determines the chemical properties of an organic compound.
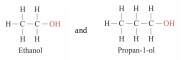
have similar chemical properties because they contain the same functional group –OH. They are classified into the same homologous series — Alcohols.
Family
|
General formula |
Functional group |
Specific example |
|
Formula |
IUPAC name |
|||
Alkane |
RH |
C – H and C –C bonds |
CH3CH3 |
Ethane |
Alkene |
RCH = CH2 RCH = CHR R2C = CHR R2C = CR2 |
|
CH2 = CH2 |
Ethene |
Alkyne |
RC ≡ CH RC ≡ CR |
– C ≡ C – |
HC≡CH |
Ethyne |
Aromatic hydrocarbon |
ArH |
Aromatic ring
|
|
Benzene |
Haloalkane |
RX |
|
CH3Cl |
Chloromethane |
Alcohol
|
ROH |
|
CH3OH |
Methanol |
![]()
Family
|
General formula |
Functional group |
Specific example |
|
Formula |
IUPAC name |
|||
Ether |
R – O – R |
|
CH3 – O – CH3 |
Methoxymethane |
Aldehyde |
|
|
|
Methanal |
Ketone |
|
|
|
Propanone |
Carboxylic acid |
|
|
|
Ethanoic acid |
Amine |
RNH2 R2NH R3N |
|
CH3NH2 |
Methylamine |
Nitrile |
RC ≡ N |
– C ≡ N |
CH3CN |
Ethanenitrile |
Ester |
|
|
|
Methyl ethanoate |
Acyl halide |
|
|
|
Ethanoyl chloride |
Amide |
|
|
|
Ethanamide |
Acid anhydride |
|
|
|
Ethanoic anhydride |
Members in the same series can be represented by a general formula.
e.g. General formula of alkanes: CnH2n+2
General formula of alcohols: CnH2n+1OH
A homologous series is a series of compounds that have the same functional group, and each member differs from the next member by a – CH2 – unit in their formulae. Members of a homologous series have similar chemical properties
The physical properties change gradually along the homologous series e.g. the longer the carbon chain in molecule ( or the greater the molecular mass)
- the greater the attractive force between molecules
- the higher the melting point and boiling point
