
- •3. What is Radioactivity?
- •1. Ionizing radiation
- •2. Radioactive Elements
- •3. The Nature of Radiation
- •4. Alpha radiation
- •5. Beta and gamma radiation
- •6. What is an isotope?
- •7. The Radioactive Series
- •8. The Energy of the Radiation
- •8.1. Description of a Radioactive Source
- •8.2. Alpha Radiation
- •8.3. Beta Radiation
- •8.4. Gamma Radiation
- •9. The Penetration of Radiation
- •10. Ionization. Excitation.
- •Control points to Charter 3
- •Science vocabulary
10. Ionization. Excitation.
Ionizing radiation is a more precise name for all types of radiation with energy large enough to ionize a molecule (below, M = molecule). Included under this designation are types of radiation from radioactive sources (α-, β- and γ-rays), x-rays, short wave length UV, particles from accelerators, particles from outer space, and neutrons.
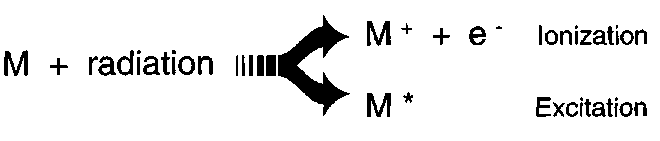
Most atoms and molecules have ionization energy of 10eV and more. Certain molecules in liquids and іn the solid state may have ionization energy as low as 6 eV. This means that UV-radiation with a wavelength below approximately 200 nm (6.2 eV) may cause ionization. Radiation with energy of 1 MeV has enough energy to yield about 150,000 ionizations if all the energy deposited produces ions.
The electron which is ejected from the molecule in an ionization process is called a secondary electron. Secondary electrons with a starting energy of 100 eV or more make their own tracks and will ionize and excite other molecules. These electrons are called delta rays.
Ionizing radiations not only ionize but can also excite molecules. Excitations are also produced by long wavelength UV and visible light (called non-ionizing radiation). An excitation occurs when the molecule attains extra energy. This is done by increasing the vibrational, rotational, or electronic energies of the molecule. These excited states have short life times (less than milliseconds) and sometimes relax back to the ground state by emitting light. Light emission from an excited molecule, called fluorescence and phosphorescence, is a property that is used to measure and characterize ionizing radiation.
Excited and ionized molecules are very reactive and have short life times. These reactive products represent the starting point for all radiobiological effects, such as cancer. The biological effect increases with the number of ions and excited molecules formed.
Control points to Charter 3
What is ionizing radiation?
Radioactivity.
The law of radioactive decay.
α-particles and Alpha radiation.
What is the difference between Beta and Gamma radiation?
What is an isotope?
Radioactive series; half-lives of radioactive series.
The unit for radiation energy. Radioactive source.
Alpha radiation. LET.
Beta radiation. The rule of thumb.
Gamma radiation. Photoelectric effect. Compton scattering. Pair production.
Penetration of radiation (α-particles, β-particles, γ-particles).
What is the difference between ionization and excitation?
Science vocabulary
1. disintegration розпадання; подрібнення на складові частини
2. atomic weight атомна маса
3. nucleon нуклон
4. attain досягати
5. deuterium дейтерій, важкий водень
6. eject вивергати; викидати
7. radioactive series радіоактивний ряд
8. penetrate проникати всередину
9. dissipate розсіювати(ся)
10. radioactive decay радіоактивний розпад
11. trace out креслити, накреслювати
12. linear track лінійний трек
13. energy gap перепад енергії; енергетичний інтервал
14. tiny дуже маленький, крихітний
15. rule of thumb практичний метод; емпіричне правило
16. collision зіткнення
17. quantum (a) квант
18. photoelectric effect фотоелектричний ефект
19. inelastic scattering непружне розсіювання
20. pair production утворення (електронно-позитронних) пар
21. impart надавати; передавати
22. secondary electron вторинний електрон
23. pellet кулька
24. half-value layer шар половинного послаблення (іонізуючого випромінювання)
25. nm (nanometre) нанометр
26. fluorescence флуоресценція, свічення
27. phosphorescence фосфоресценція, свічення
