
- •1 Introduction
- •2 Nomenclature of Halogeno-compounds
- •3 Physical Properties of Halogeno-compounds
- •4 Preparation of Halogeno-compounds
- •5 Reactions of Halogeno-compounds
- •6 Nucleophilic Substitution Reactions
- •1. Sn2 reactions
- •2. Sn1 reactions
- •1. Experiment 1 : Comparison of the rates of hydrolysis of 1-chlorobutane, 1-bromobutane and 1-iodobutane
- •2. Experiment 2: Comparison of the rates of hydrolysis of primary, secondary and tertiary haloalkanes and halobenzene
- •7 Elimination Reactions
- •8 Uses of Halogeno-compounds
6 Nucleophilic Substitution Reactions
Reaction with Sodium Hydroxide
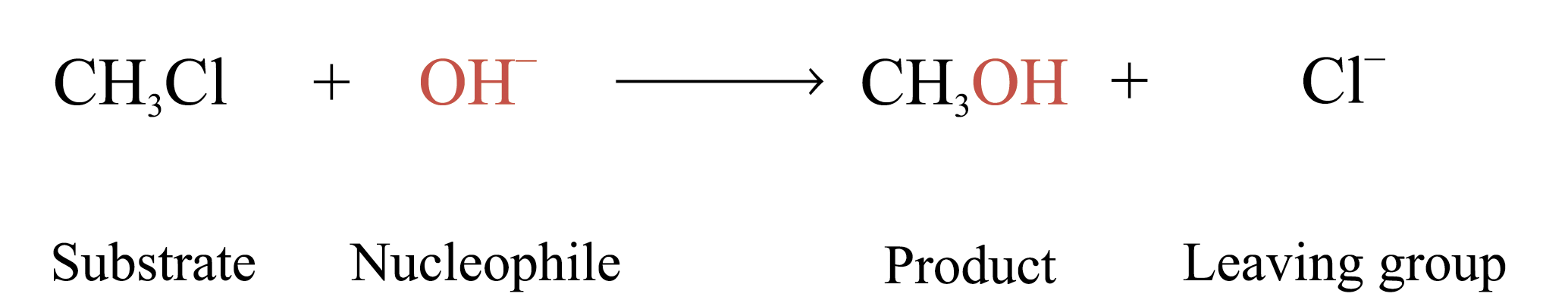
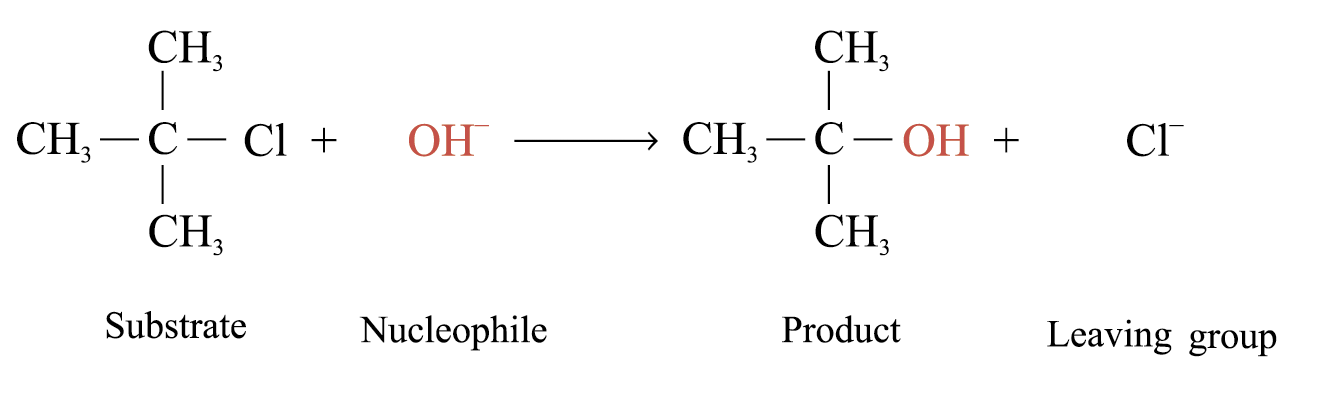
The reactions proceed in 2 different reaction mechanisms: bimolecular nucleophilic substitution (SN2)
unimolecular nucleophilic substitution (SN1)
Bimolecular Nucleophilic Substitution (SN2)
Example: CH3 – Cl + OH– ®¾ CH3OH + Cl–
Rate = k[CH3Cl][OH–]
Order of reaction = 2 Þ both species are involved in rate determining step
Reaction mechanism of the SN2 reaction:
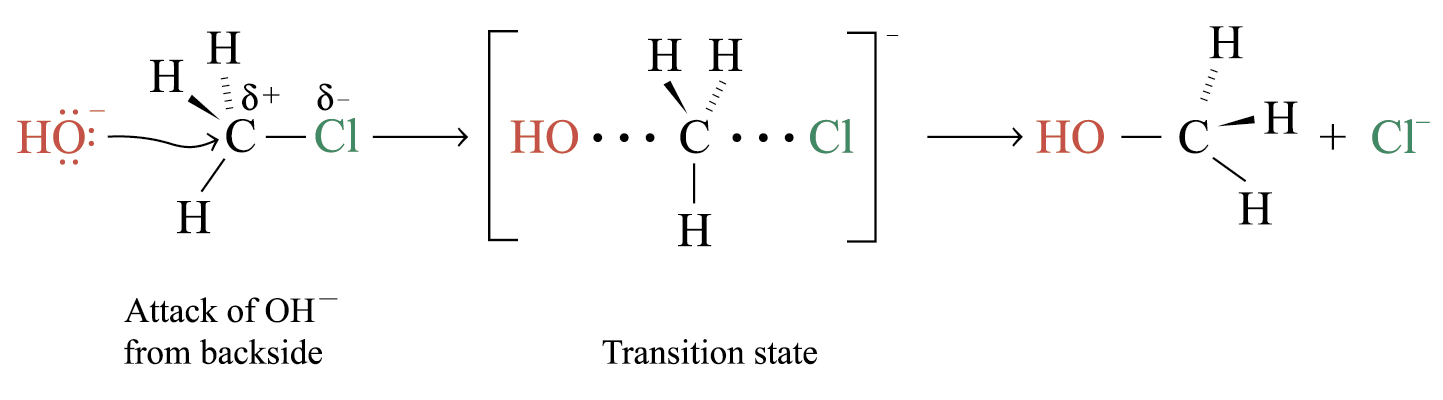
The nucleophile attacks from the backside of the electropositive carbon centre
In the transition state, the bond between C and O is partially formed, while the bond between C and Cl is partially broken
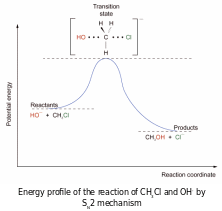
Transition state involve both the nucleophile and substrate Þ second order kinetics of the reaction
Stereochemistry of SN2 Reactions
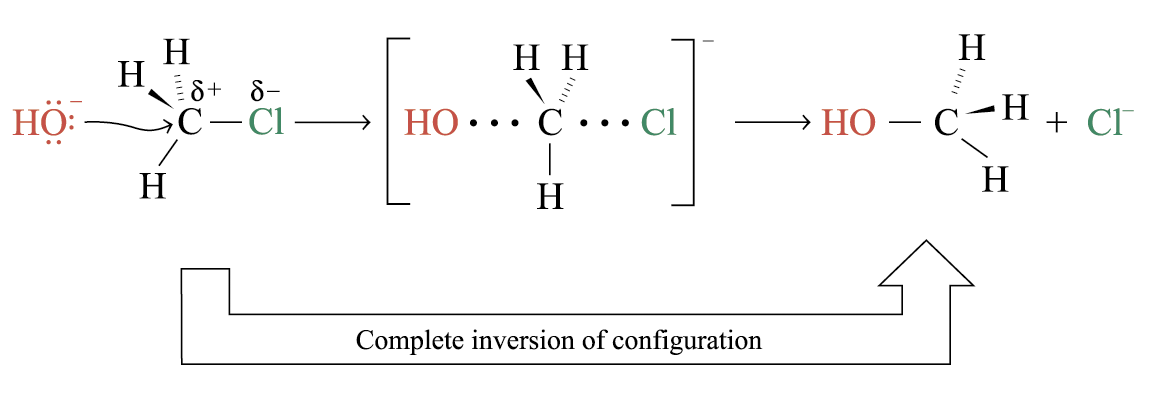
The nucleophile attacks from the backside of the electropositive carbon centre
The configuration of the carbon atom under attack inverts
Unimolecular Nucleophilic Substitution (SN1)
Example:
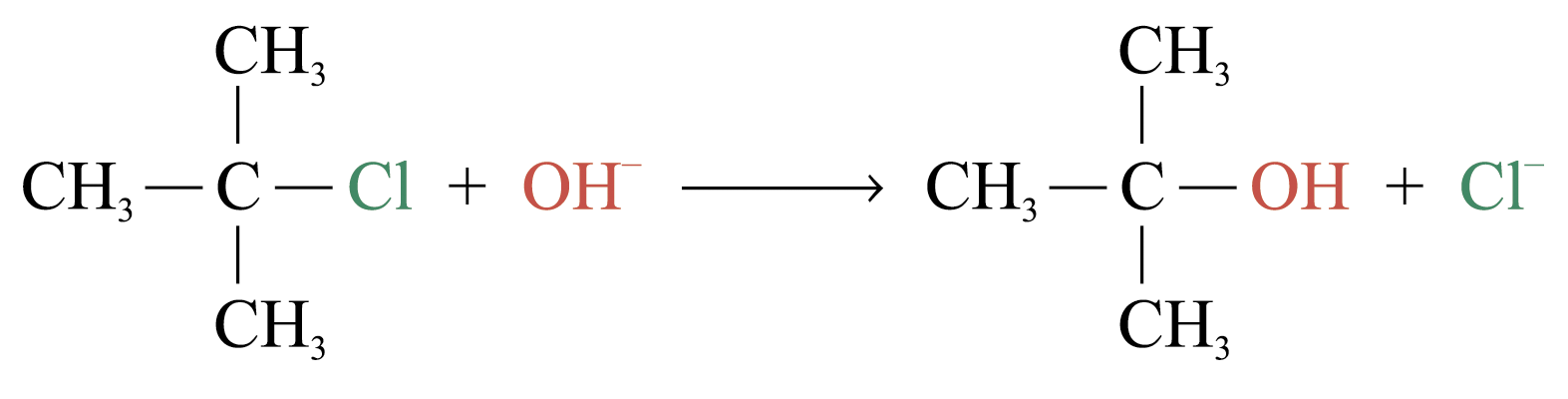
Kinetic study shows that: Rate = k[(CH3)3CCl]
The rate is independent of [OH–]
Order of reaction = 1 Þ only 1 species is involved in the rate determining step
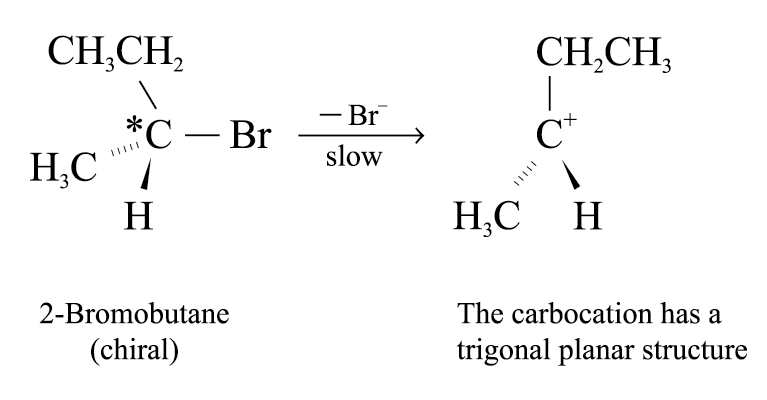
Reaction mechanism of SN1 reaction involves 2 steps and 1 intermediate formed
Step 1:
Slowest step (i.e. rate determining step)
Formation of carbocation and halide ion
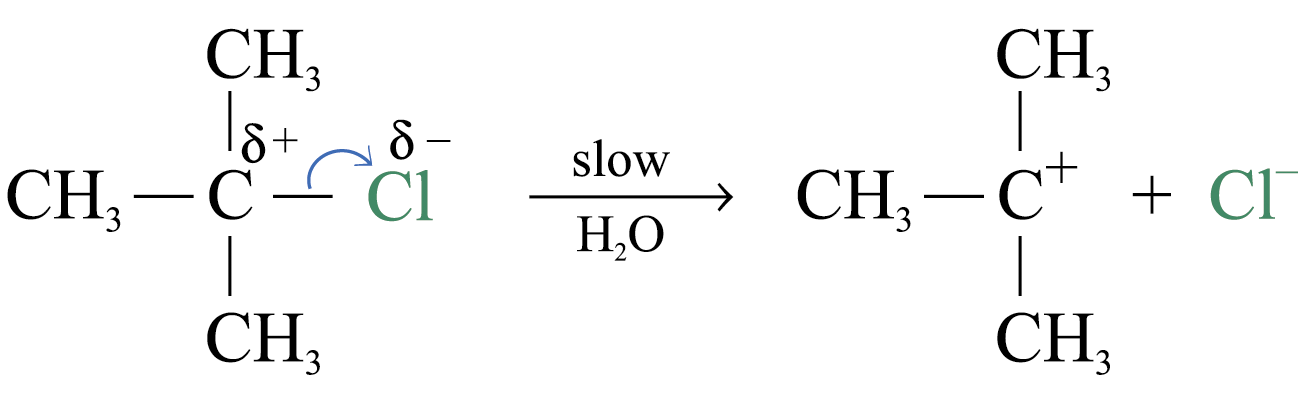
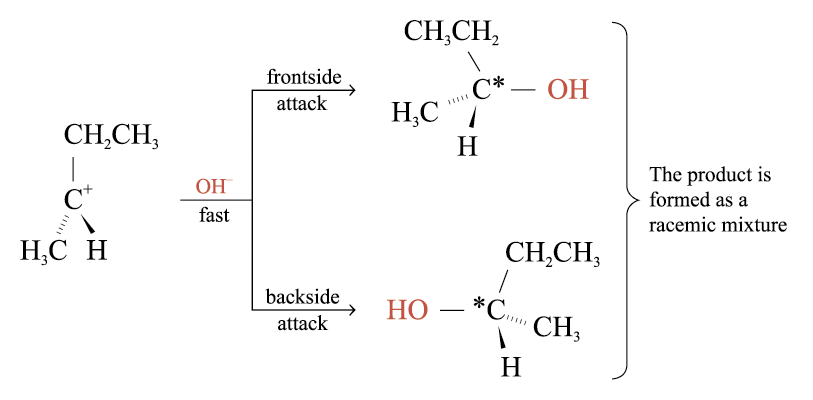
Step 2:
Fast step
Attacked by a nucleophile to form the product

Rate determining step involves the breaking of the C – Cl bond to form carbocation
Only 1 molecule is involved in the rate determining step Þ first order kinetics of the reaction
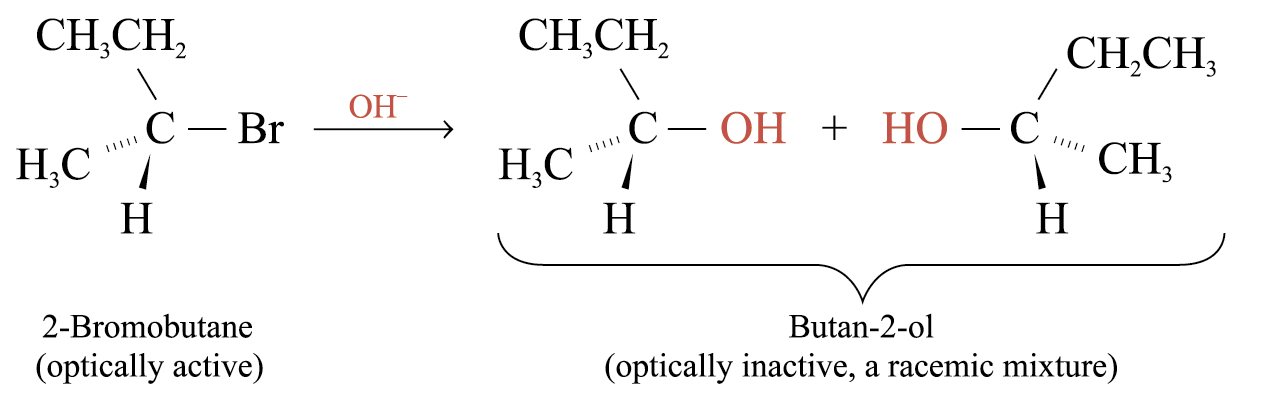
Stereochemistry of SN1 Reactions
The carbocation formed has a trigonal planar structure
The nucleophile may either attack from the frontside or the backside
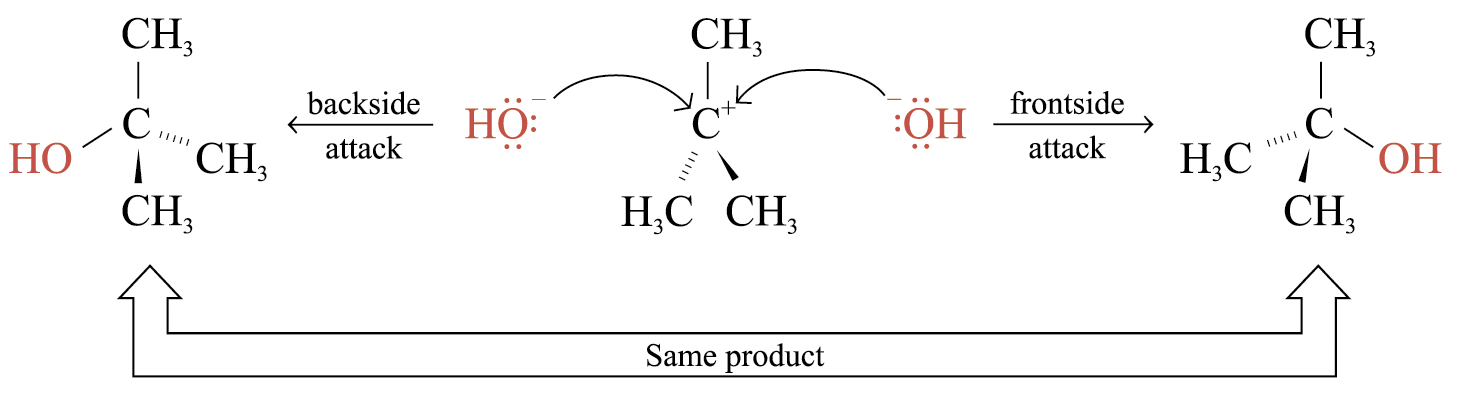
For some cations, different products may be formed by either mode of attack
The reaction is called racemization
The above SN1 reaction leads to racemization. Formation of trigonal planar carbocation intermediate
The attack of the nucleophile from either side of the planar carbocation occurs at equal rates and results in the formation of the enantiomers of butan-2-ol in equal amounts
Factors Affecting the Rates of SN1 and SN2 Reactions:
Most important factors affecting the relative rates of SN1 and SN2 reactions:
1. The structure of the substrate
2. The concentration and strength of the nucleophile (for SN2 reactions only)
3. The nature of the leaving group
The Structure of the Substrate
1. Sn2 reactions
The reactivity of haloalkanes in SN2 reactions: CH3X > 1° haloalkane > 2° haloalkane > 3° haloalkane
Steric hindrance affects the reactivity bulky alkyl groups will inhibit the approach of nucleophile to the electropositive carbon centre Þ energy of transition state Þ activation energy
Steric effects in the SN2 reaction
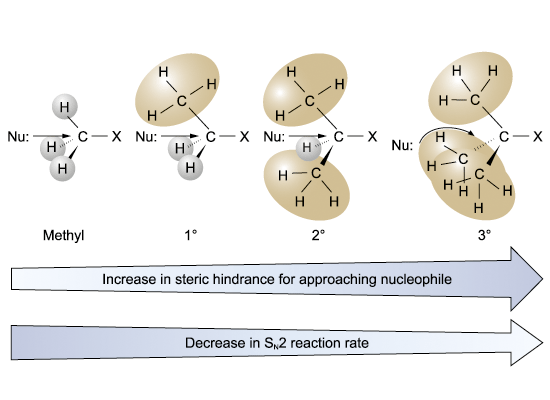
2. Sn1 reactions
Critical factor: the relative stability of the carbocation formed
Tertiary carbocations are the most stable 3 electron-releasing alkyl groups stabilize the carbocation by releasing electrons
Methyl, 1°, 2° carbocation have much higher energy Þ activation energies for SN1 reactions are very large and rate of reaction become very small
The Concentration and Strength of the Nucleophile
Only affect SN2 reactions
Concentration of nucleophile Þ rate
Relative strength of nucleophiles can be correlated with two structural features:
(I) A negatively charged nucleophile (e.g. OH–) is always a stronger nucleophile than a neutral nucleophile (e.g. H2O)
(II) In a group of nucleophiles in which the nucleophilic atom is the same, the order of nucleophilicity roughly follows the order of basicity:
e.g. RO– > OH– >> ROH > H2O
Strength Þ rate
The Nature of Leaving Group
Halide ion departs as a leaving group
For the halide ion, the ease of leaving: I– > Br– > Cl– > F–
This is in agreement with the order of bond enthalpies of carbon-halogen bonds
Uncharged or neutral compounds are better leaving groups e.g. The ease of leaving of oxygen compounds:
H2O >> OH– > RO–
Strongly basic ions rarely act as leaving group e.g.
![]()
When an alcohol is dissolved in a strong acid, it can react with a halide ion. The acid protonates the –OH group, and the leaving group becomes a neutral water molecule
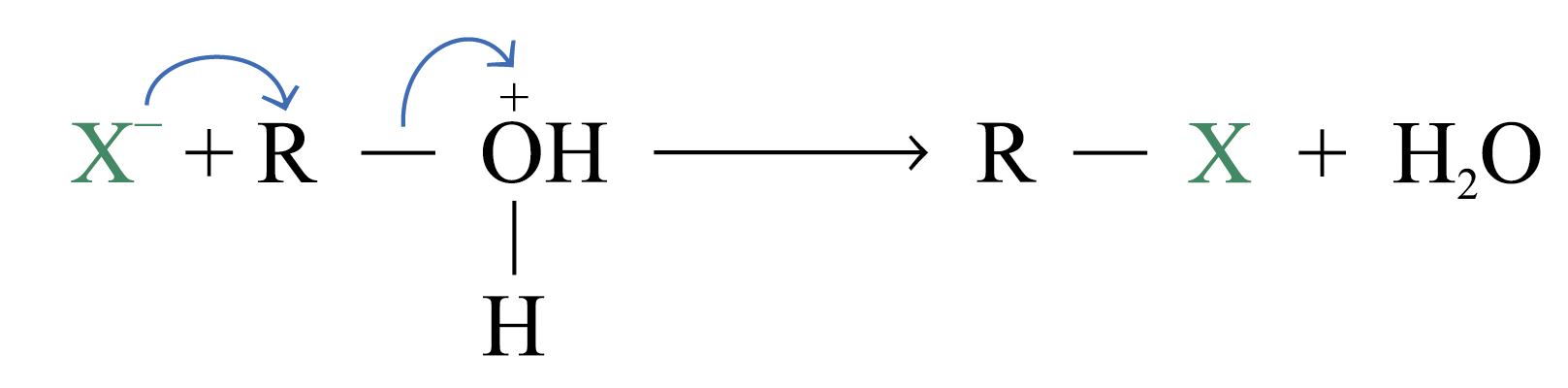
Comparision of Rates of Hydrolysis of Haloalkanes and Halobenzene
