
A rendiazonium Salts
Primary arylamines react with nitrous acid, HNO2, to yield stable arenediazonium salt
Arendiazonium salts are extremely useful in synthesis because the diasonio group (N2+) can be replaced by nucleophiles in asubstitution reaction
![]()
Preparation of Substituted Aromatic Compounds by Diazonio Replacement Reactions
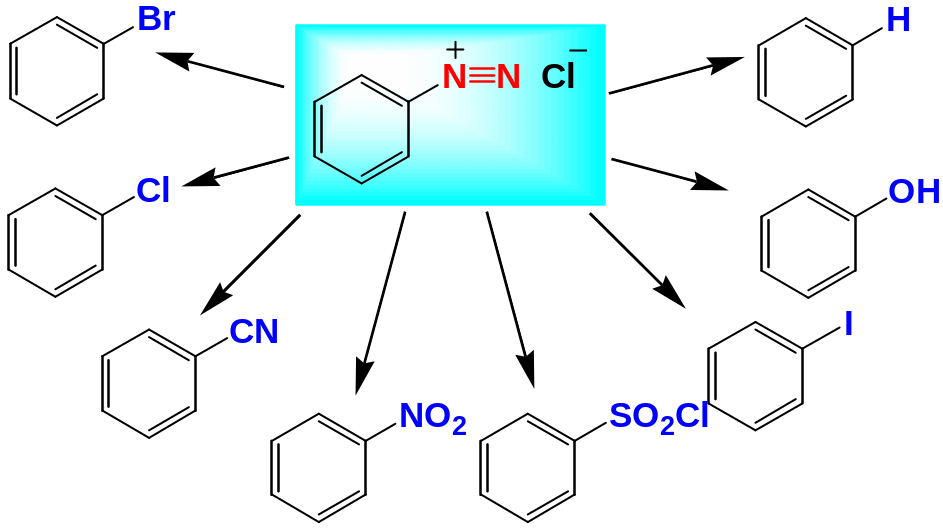
Replacement of the Diazonium Group by –Cl, –Br or –CN (The Sandermeyer Reaction)
The diazonium group of benzenediazonium ions can be replaced by –Cl, –Br and –CN by treating with CuCl, CuBr and CuCN respectively
![]() ,
,
![]()
![]()
Replacement of the Diazonium Group by –NO2
The diazonium group of benzenediazonium ions can be replaced by –NO2 by treating with NaNO2 in the presence of catalysts such as CuNO2

Replacement of the Diazonium Group by –SO2Cl
The diazonium group of benzenediazonium ions can be replaced by –SO2Cl on reacting with SO2 in the presence of catalysts such as CuCl
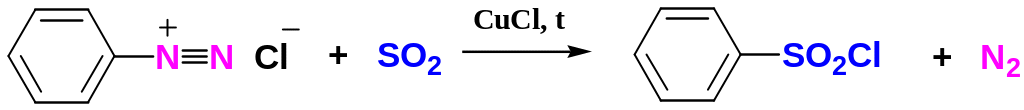
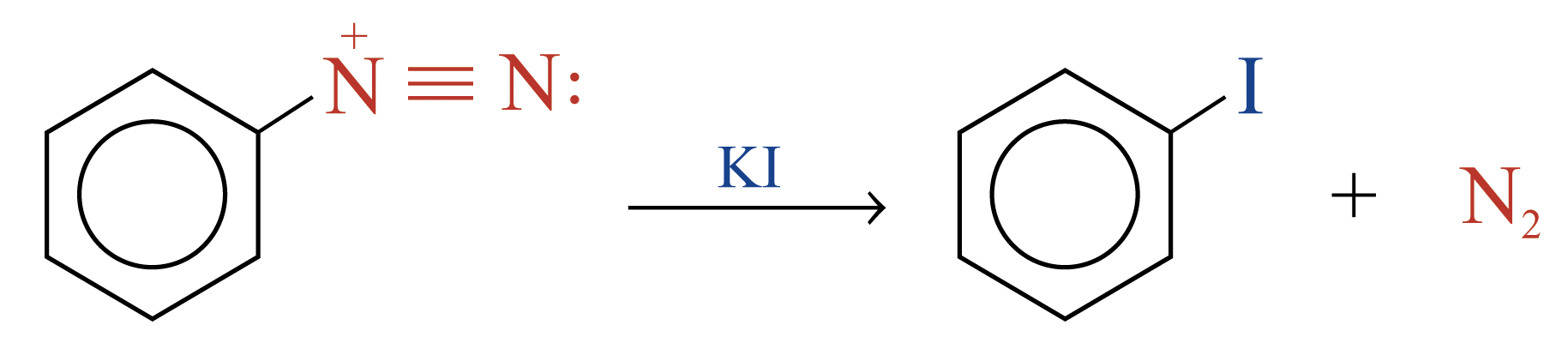
Replacement of the Diazonium Group by –I
The diazonium group of benzenediazonium ions can be replaced by –I on reacting with KI
Replacement of the Diazonium Group by –F
The diazonium group of benzenediazonium ions can be replaced by –F on reacting with KBF4
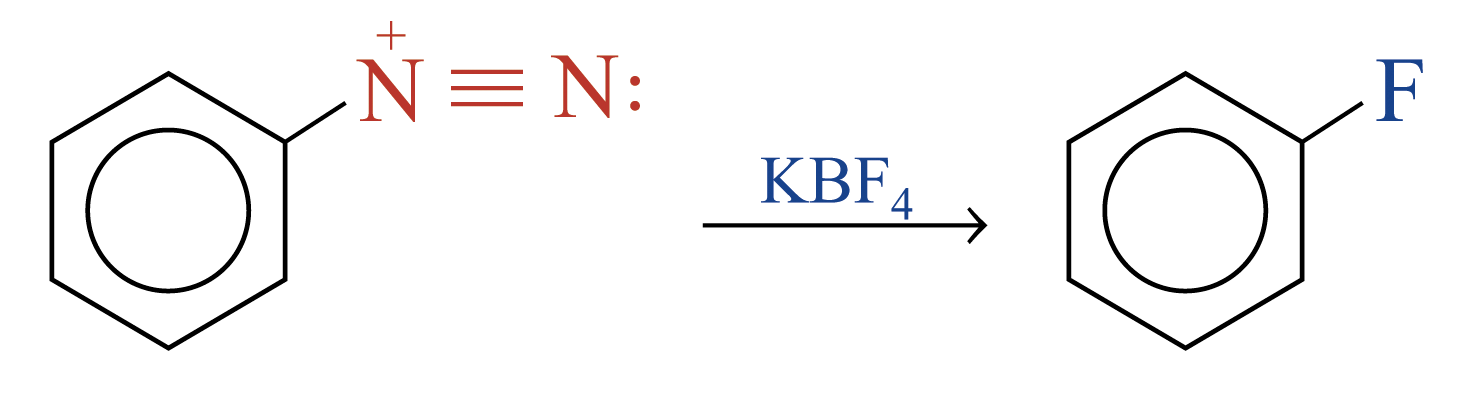
Replacement of the Diazonium Group by –OH
The diazonium group of benzenediazonium ions can be replaced by –OH to yield phenols by addition of the arenediazonium salt to hot aqueous acid:
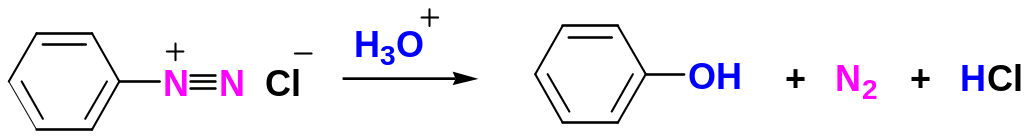
Replacement of the Diazonium Group by –H
The diazonium group of benzenediazonium ions can be replaced by –H to yield arenes by reduction of the arenediazonium salt with hypophosphorous acid:
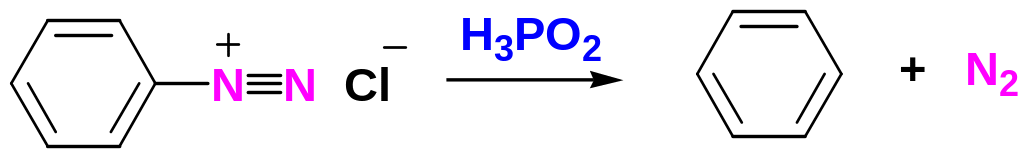
Coupling Reactions of Benzenediazonium Ions
Benzenediazonium ions react with highly reactive aromatic compounds in an alkaline medium to give azo compounds
Example:
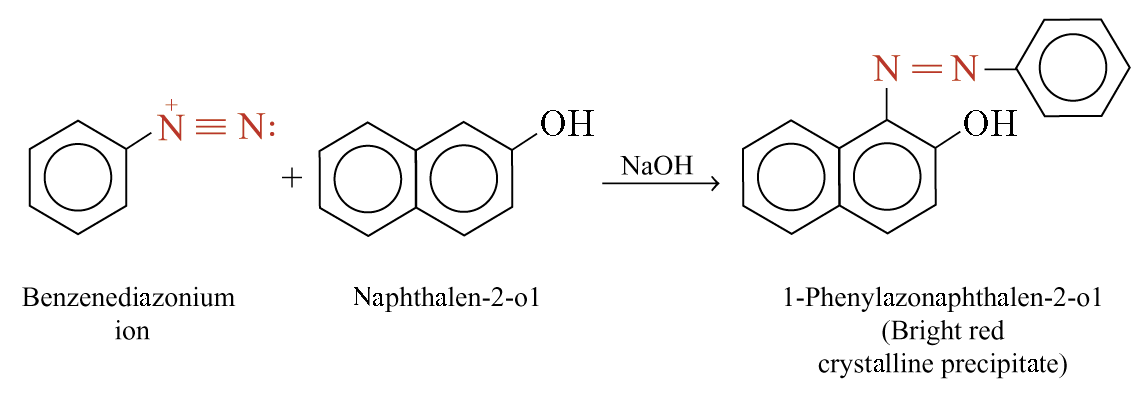
Benzenediazonium ions react with highly reactive aromatic compounds in an alkaline medium to give azo compounds
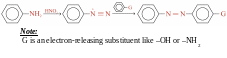
Diazonium coupling reactions are typical electrophilic aromatic substitutions in which the positively charged diazonium ion is the electrophile that reacts with the electron-rich ring of a phenol or arylamine.
Reaction usually occurs at the para position, although orto attack can take place if the para position is blocked
Example:
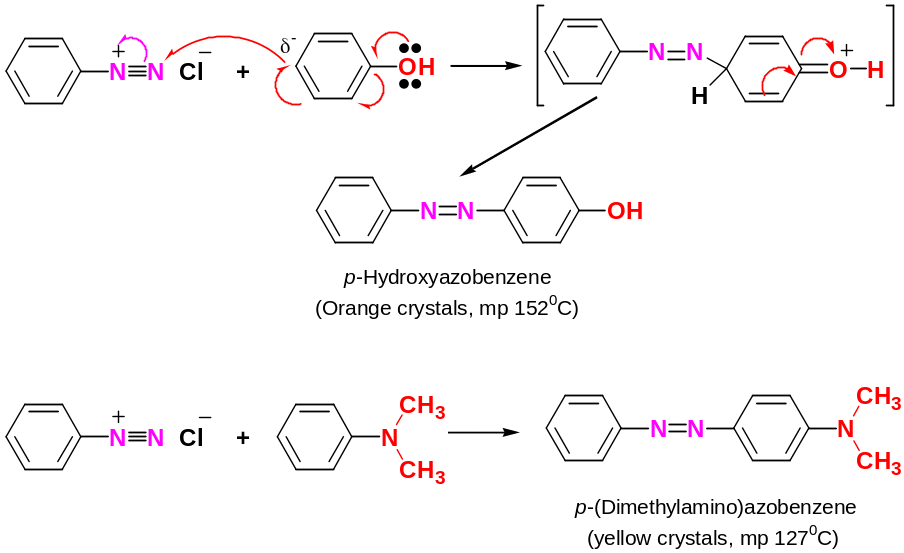
This reaction is used to identify 1° aromatic amines
Azo compounds are usually intensely coloured – N = N – link brings the two aromatic rings into conjugation, and the p electrons are delocalized over the entire structure
Azo compounds are used as dyes due to intense colours
Uses of Amines and their Derivatives
Azo compounds are highly coloured and can be synthesized from relatively inexpensive compounds Þ widely used in dyeing industry
Examples:
1. Methyl orange (an orange dye for fabrics)

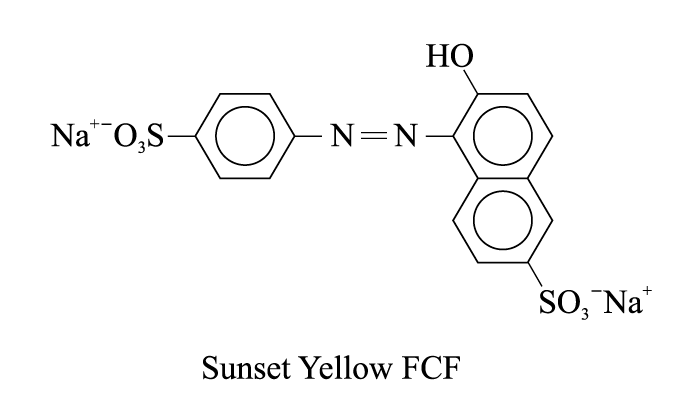
2. Sunset Yellow FCF (an orange-yellow dye for food products)
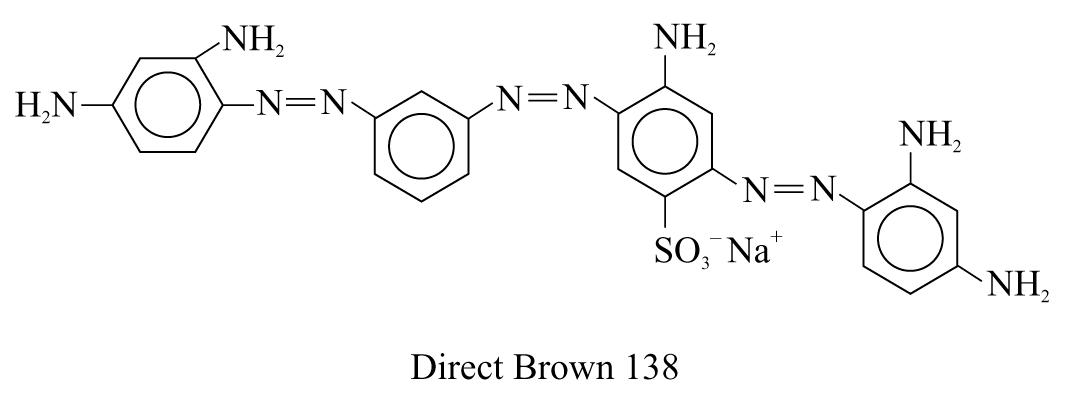
3. Direct Brown 138 (a brown dye for fabrics)
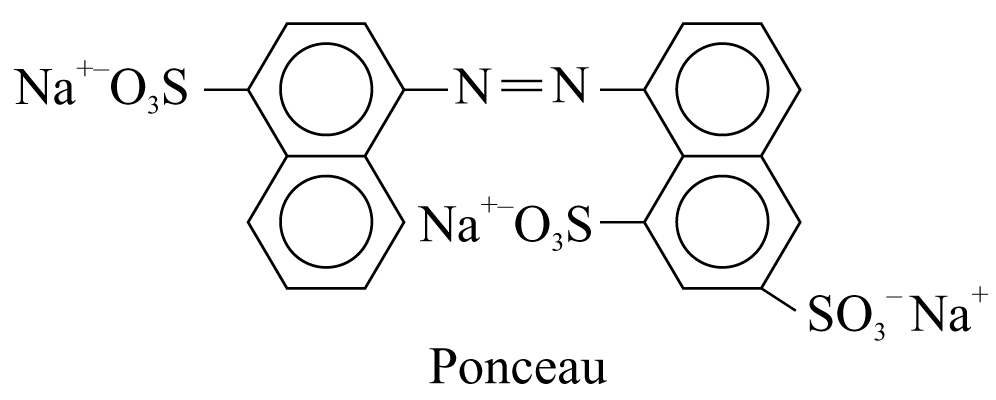
4. Ponceau (a red dye for food products)
As Drugs
Amine derivatives are commonly used as drugs such as painkillers (analgesics), transquillizers and antihistamines
1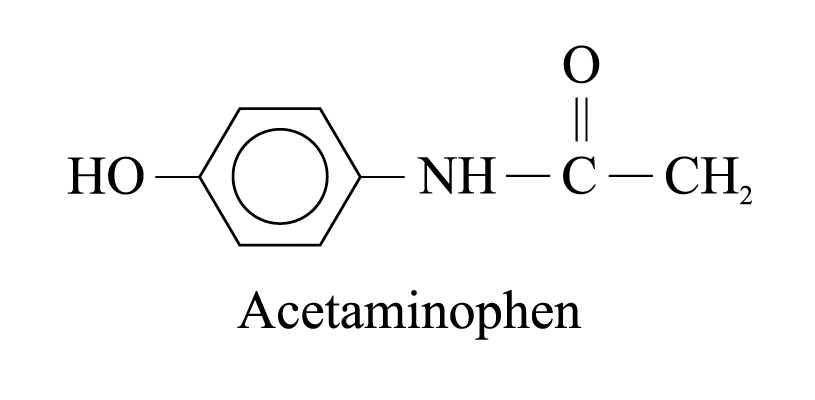 . Acetaminophen
(also known as paracetamol)
. Acetaminophen
(also known as paracetamol)
used to relieve pains
less harmful to the stomach compared with aspirin
2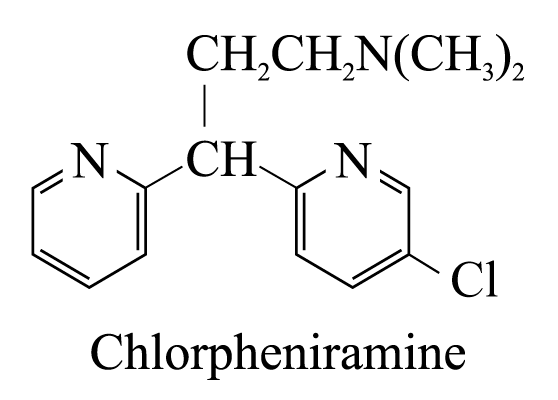 . Chlorpheniramine
. Chlorpheniramine
helps to relieve allergic disorders caused by cold, insect bites and stings
p
 resent
in some over the counter drugs such as Coricidin, Coltaline, Piriton
and Dristan
resent
in some over the counter drugs such as Coricidin, Coltaline, Piriton
and Dristan
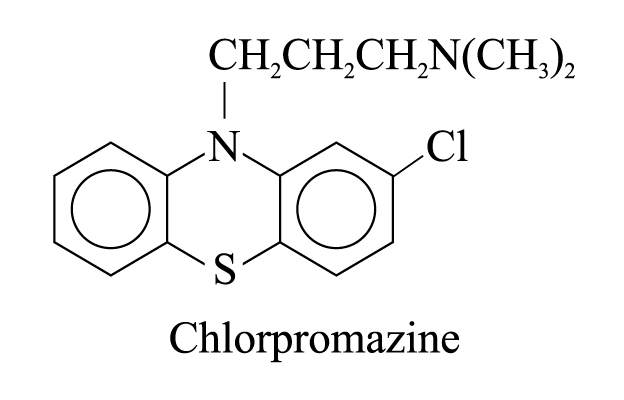
3. Chlorpromazine
sedative effect without inducing sleep
used to relieve anxiety, excitement, restlessness and even metal disorders
Контрольні питання до лекції 7.
Напишіть структурні формули сполук. Розташуйте сполуки за збільшенням їх основності. Дайте пояснення. П-хлоранілін, 2-бром-4-хлоранілін, о-толуїдин
Вкажіть правильну схему синтезу трет-бутанолу з ізобутилену. Напишіть реакції. А)
 ;
В)
;
В)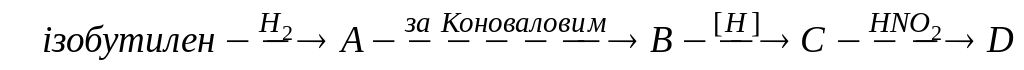 ;
С)
;
С)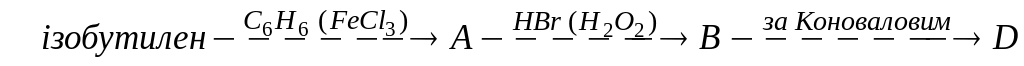 ;
D)
;
D) .
.Встановити будову сполуки за брутто-формулою та продуктами хімічного перетворення: С7Н9N , з соляною кислотою утворює сіль, після послідовної взаємодії з нітритною кислотою і нітритом калію в присутності міді утворює п-нітротолуол. Напишіть реакції.
Запропонуйте схему синтезу м-метилфенолу з хлорбензолу
З
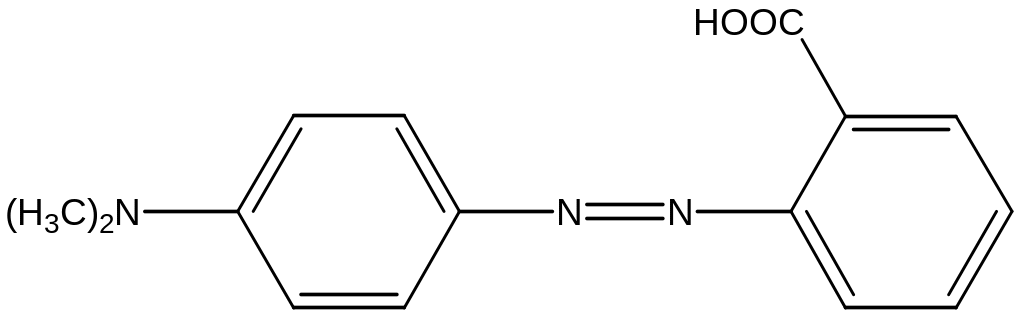 апропонуйте
схему синтезу барвника з азо- і
діазокомпоненти. Назвіть сполуки.
апропонуйте
схему синтезу барвника з азо- і
діазокомпоненти. Назвіть сполуки.
