
- •1 Alkanes
- •1.1 Nomenclature of Alkanes
- •1.2 Physical Properties of Alkanes
- •1.3 Preparation of Alkanes
- •Industrially Preparation
- •1.4 Reactions of Alkanes
- •3. Chain termination
- •1.5 Cycloalkanes
- •1.6 Nomenclature of Cycloalkanes
- •1.7 Isomerization of Cycloalkanes
- •1.8 Reactions of Cycloalkanes
- •2 Alkenes
- •2.1 Nomenclature of Alkenes
- •2.2 Physical Properties of Alkenes
- •2.3 Preparation of Alkenes
- •2.4 Reactions of Alkenes
2.2 Physical Properties of Alkenes
Name |
Formula |
Boiling point (°C) |
Melting point (°C) |
Density at 20 °C (g cm-3) |
Ethene |
CH2 = CH2 |
-104 |
-169 |
— |
Propene |
CH3CH = CH2 |
-47.7 |
-185 |
0.514 |
But-1-ene |
CH3CH2CH = CH2 |
-6.3 |
-185 |
0.595 |
Pent-1-ene |
CH3(CH2)2CH = CH2 |
30 |
-165 |
0.641 |
Hex-1-ene |
CH3(CH2)2CH = CH2 |
62.9 |
-140 |
0.673 |
cis-But-2-ene |
CH3CH = CHCH3 (cis) |
4 |
-139 |
0.621 |
trans-But-2-ene |
CH3CH = CHCH3 (trans) |
1 |
-106 |
0.604 |
2-Methylbut-1-ene |
CH3CH3C(CH3) = CH2 |
31 |
-138 |
0.650 |
2.3 Preparation of Alkenes
Cracking
alkenes can be prepared industrially by cracking of high molecular mass alkanes
![]()
![]()
Elimination Reactions
Dehydrohalogenation
Dehydrohalogenation is the elimination of a hydrogen halide molecule from a haloalkane

Examples:


The ease of dehydrohalogenation of haloalkanes decreases in the order:

Dehydrohalogenation of 2° and 3° haloalkanes can take place in more than one way and a mixture of alkenes is formed
alc.KOH CH3CH2CHClCH3 ®¾¾¾ CH3CH = CHCH3 + CH3CH2CH = CH2 heat
2-chlorobutane But-2-ene But-1-ene
(80%) (20%)
Note: the more highly substituted alkene is formed as major product
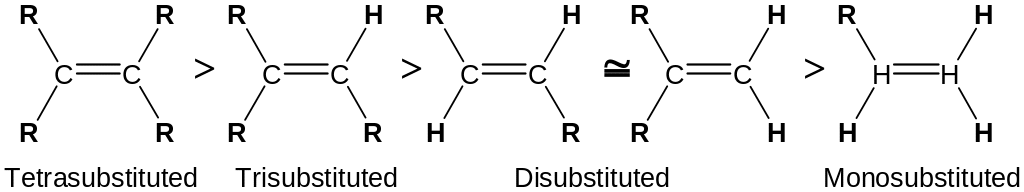
The relative stabilities of alkenes decrease in the order:
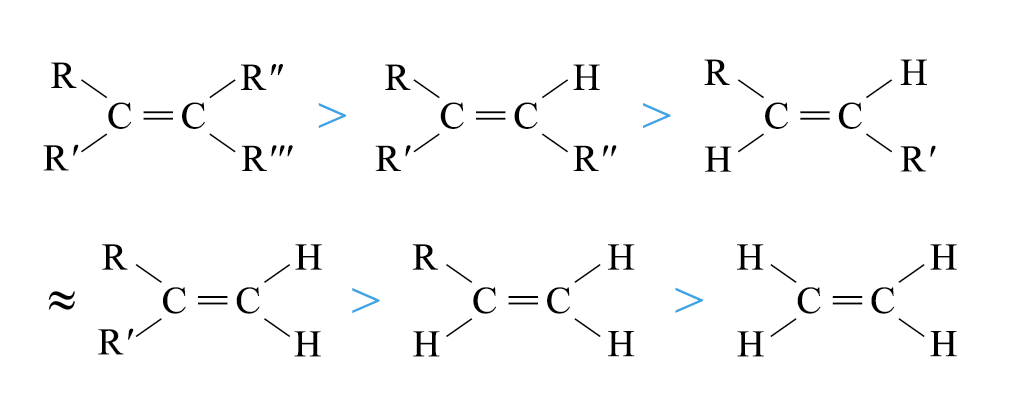
Dehydration of Alcohols

The experimental conditions of dehydration depend on the structures of alcohols
e.g.
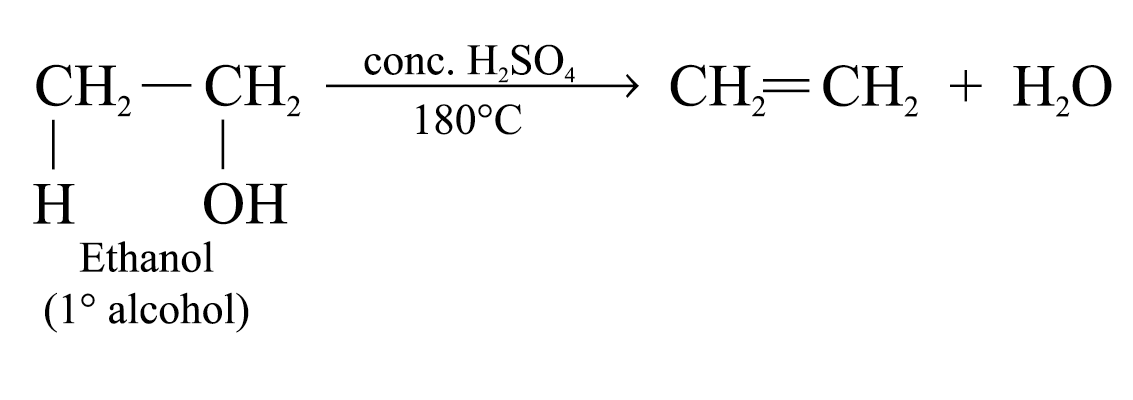
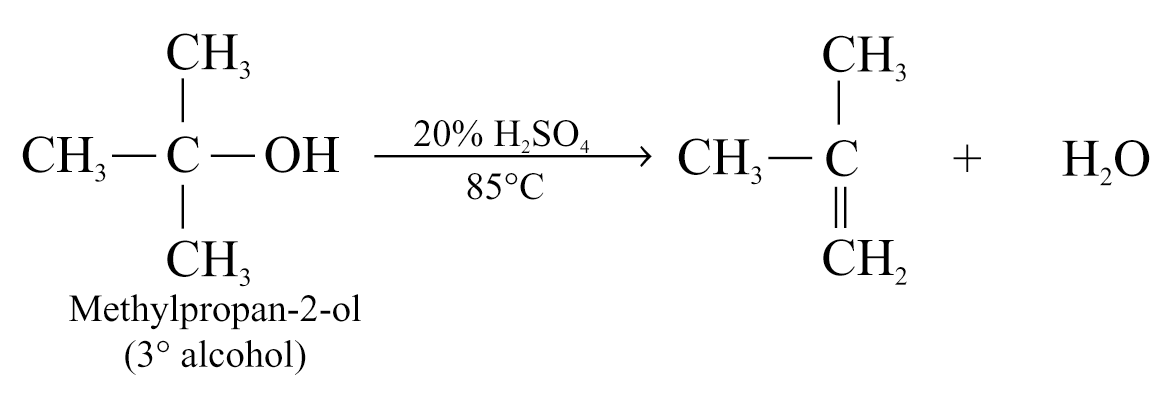
The relative ease of dehydration of alcohols generally decreases in the order:

Like dehydrohalogenation, the more highly substituted alkene is formed as the major product
2.4 Reactions of Alkenes
Addition Reactions
Hydrogenation
Нydrogenation of alkynes using Lindlar’s catalyst produces alkenes
Рrevent further hydrogenation of the alkenes formed to alkanes
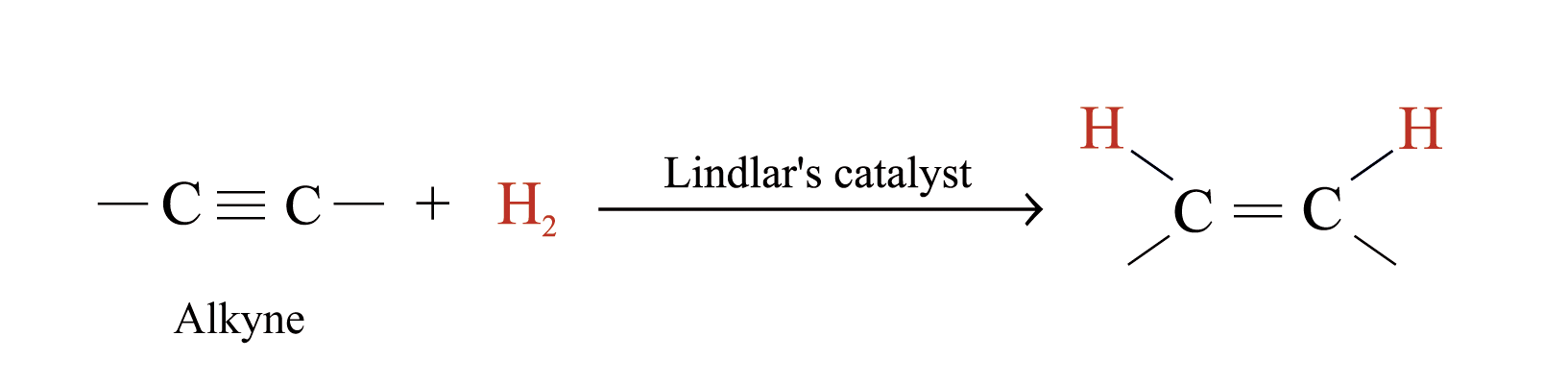
Lindlar catalyst is a finely divided palladium metal that has been precipitated onto a calcium carbonat support and then deactivated by treatment with lead acetate and quinoline, an aromatic amine.
Alkenes are more reactive than alkanes
Reason: presence of the C = C double bond
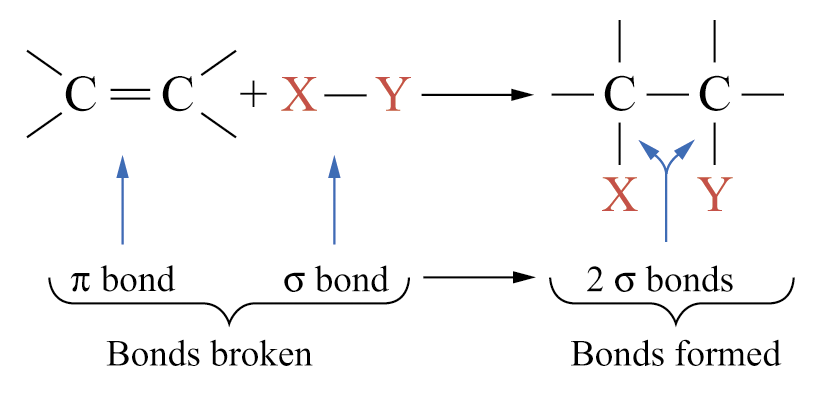

Þ alkenes undergo addition reactions and the reactions are exothermic
Further increasing the degree of substitution leads to still further stability of double bond
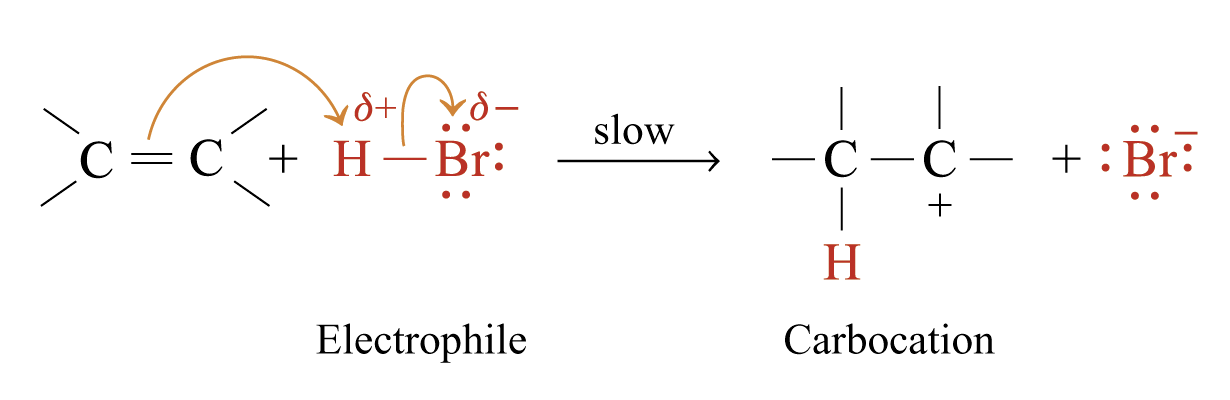
Electrons of p bond are more diffuse and less firmly held Þ susceptible to attack by electrophiles
Electrophiles such as H+, neutral reagents such as bromine (can be polarized) react with C = C double bond
Electrophilic Addition Reactions
Addition of Hydrogen Bromide

Reaction Mechanism: Electrophilic Addition Reaction of Hydrogen Bromide to Alkenes
Step 1:
Step 2:
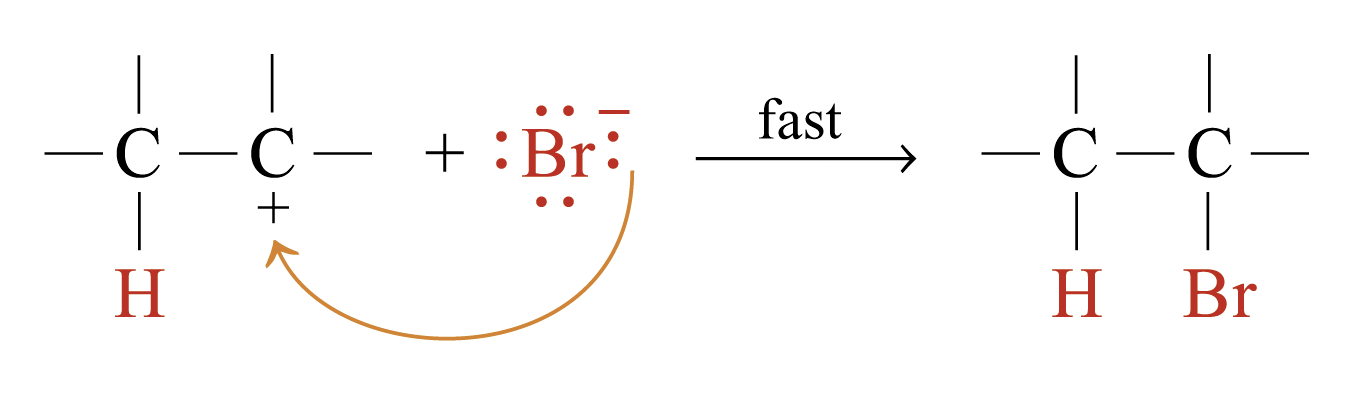
Examples:

Propene reacts with HBr to give 2-bromopropane (major product) and 1-bromopropane (minor product)
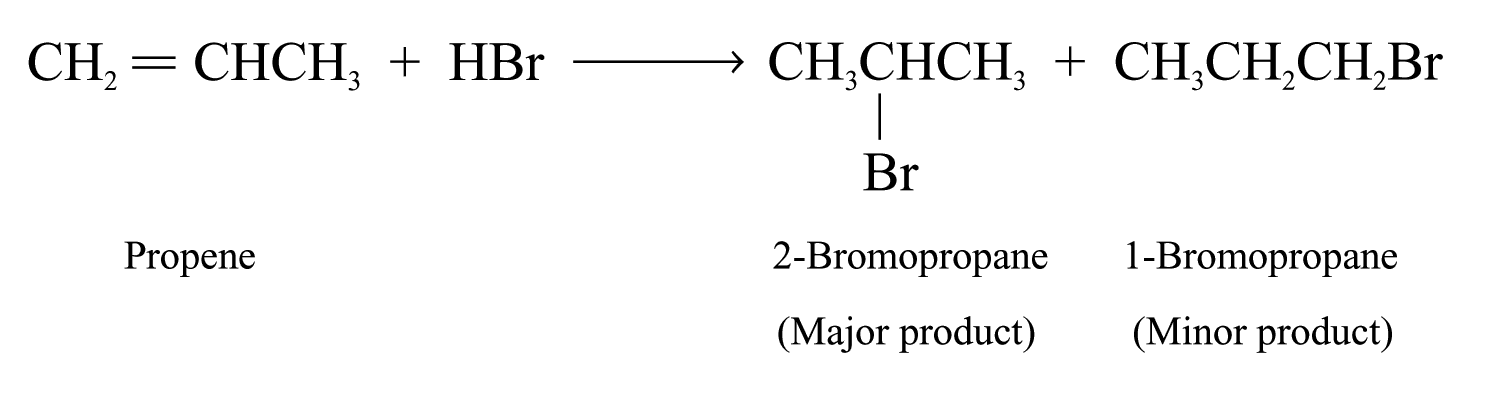
The formation of two possible products can be explained by the reaction mechanism.
Major product can be determined by the Markownikoff’s rule.
Markownikoff’s rule states that in the addition of HX to an unsymmetrical alkene, the hydrogen atom adds to the carbon atom of the carbon-carbon double bond that already has the greater number of hydrogen atoms.
Example:
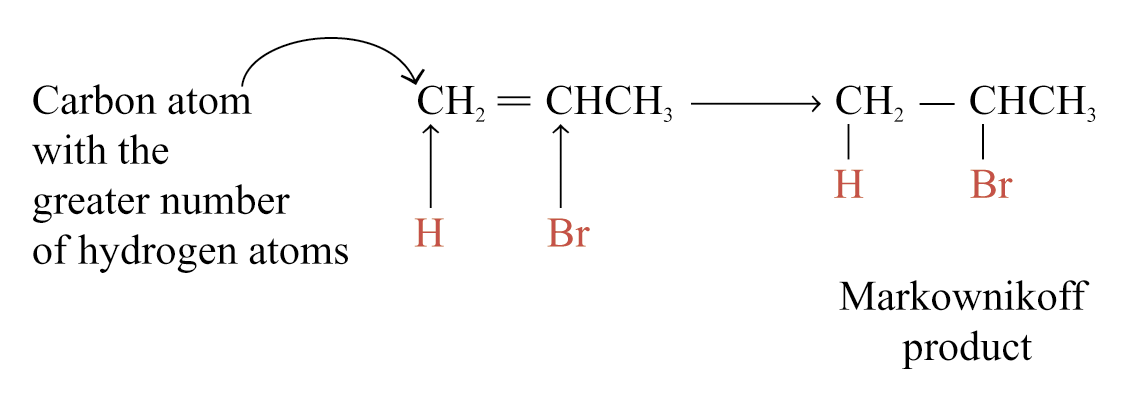
Theoretical Explanation of Markownikoff’s Rule
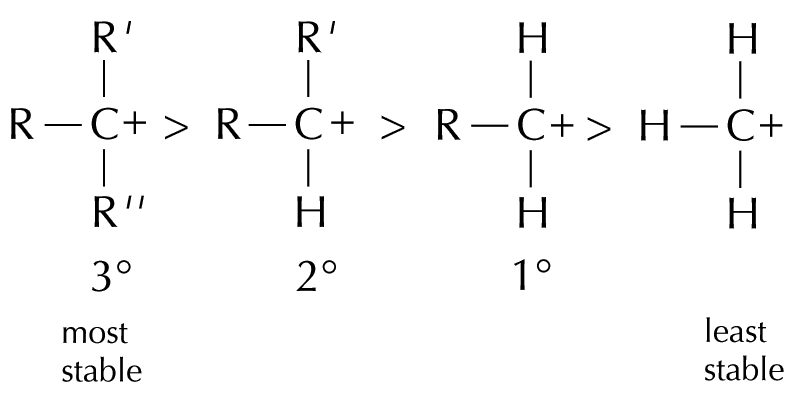
If the alkene is unsymmetrical, two different carbocations can be formed

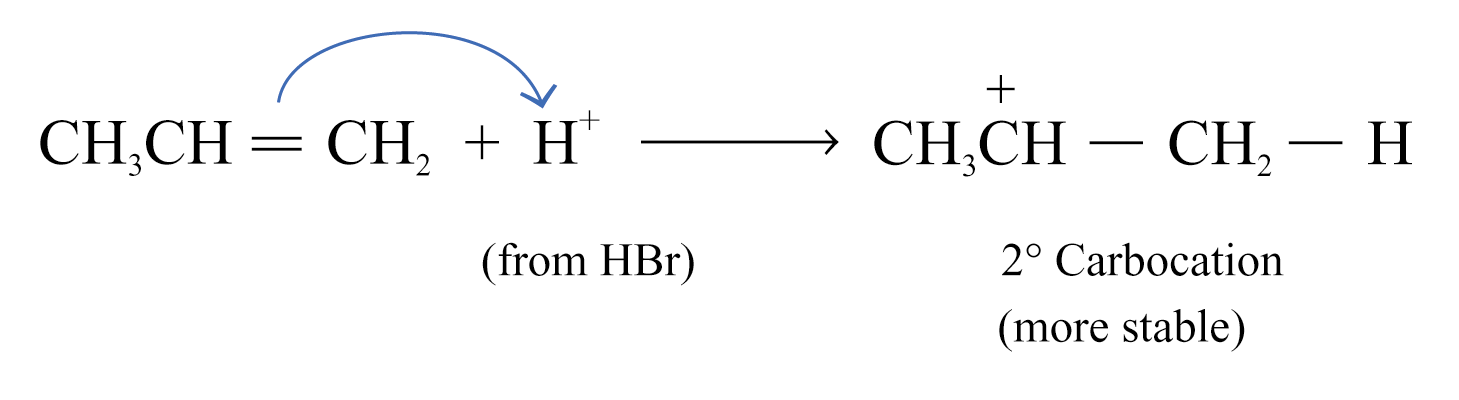
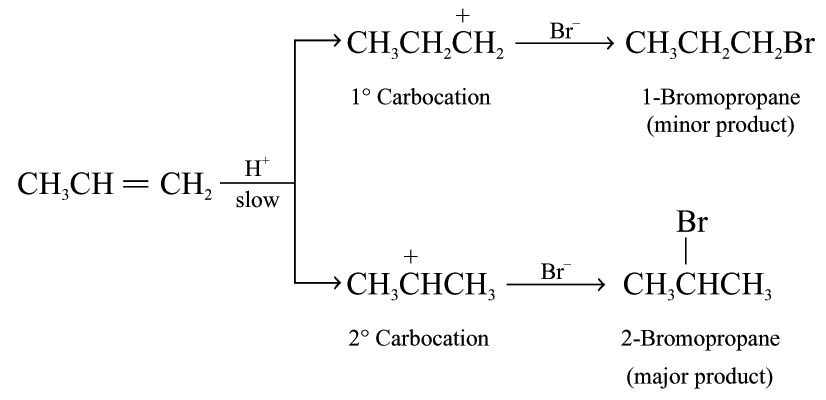
2-bromopropane is the major product because the more stable secondary carbocation is formed in the first step
Markownikoff’s rule is related to the stability of the carbocation intermediate formed in the electrophilic addition reaction.
The relative stabilities of carbocations:
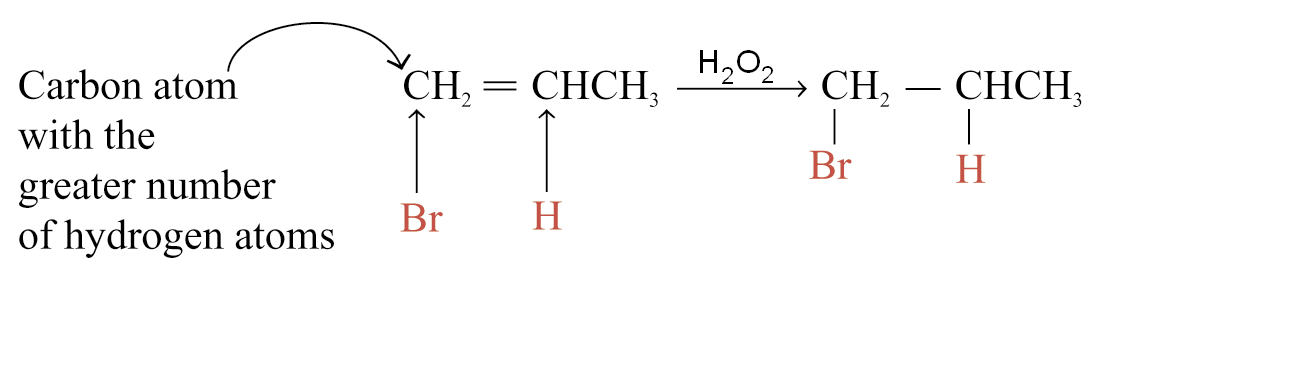
In presence peroxides Markownikoff products do not appear because a reaction mechanism is the radical addition ones
Questions and problems
Receive neohexane using catalytic hydrogenization of alkyne.
Receive 2,5-dimethylhexane using the Wurtz’s reaction (without co-products).
Receive isobutane by fusion of salt of carboxylic acid with lye.
What products can be created as a result of monochlorination of propane under the light. Arrange them in order of increasing of creation possibility. Explain. Write the reaction equations and conditions. а) chlorpropane, 2- chlorpropane; b) 2- chlorpropane, chlorpropane; с) 2-methyl-2- chlorpropane, 2- methyl -1- chlorpropane, 1-chlorbutane; d) 2- methyl -1- chlorpropane, 2- methyl -2- chlorpropane;
Which of the following compounds react with butane in the following conditions? Write the reactions. а)H2SO4 (conc), 200С; b) HNO3(conc), 200С; с) Na, 200С; d) Br2 under the light, 200С; е) KMnO4, Н2О, 200С;
Choose the methods of synthesis of 2,3-dimethylbutane from the compound, that has 4 C atoms. Write the equations. Give the name of the compound. а) catalytic hydrogenization; b) Wurtz’s reaction; с) electrolysis of water solution of salt of carboxylic acid;; d)fusion of the salt of carboxylic acid with lye; е) Cracking.
Name the compounds, that has the biggest possibility of creation in the following reactions. Write the equations.
 а) ethane,
bromethane,
butane,
chlorbutane;
b), isobutane,
2-brom-2-methylpropane,
2,2,3,3-tetramethylbutane,
2,2,3,3-tetramethyl-3-chlorbutane;
с)
propane,
2-brompropane,
2,3-dimethylbutane,
2,3-dimethyl-2-chlorbutan
;d) propane,
brompropane,
hexane,
chlorhexane.
а) ethane,
bromethane,
butane,
chlorbutane;
b), isobutane,
2-brom-2-methylpropane,
2,2,3,3-tetramethylbutane,
2,2,3,3-tetramethyl-3-chlorbutane;
с)
propane,
2-brompropane,
2,3-dimethylbutane,
2,3-dimethyl-2-chlorbutan
;d) propane,
brompropane,
hexane,
chlorhexane.
Choose the reaction that give ability to receive 3,4-dimethyl-3-chlorohexane from 1,2-dibromobutane. Name the compounds, that has the biggest possibility of creation in the following reactions. Write the equations. а)
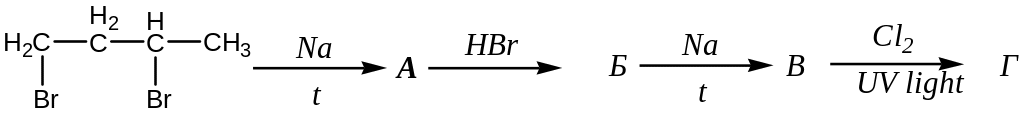 ;
b)
;
b) ;
с)
;
с)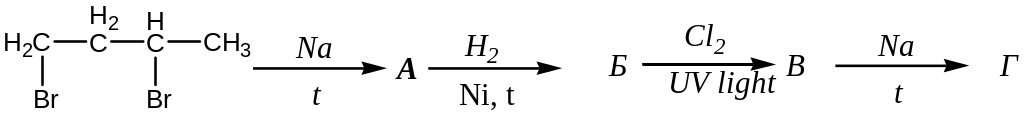 ;
d)
;
d)
Name the compounds, that has the biggest possibility of creation in the following reactions. Write the equations.
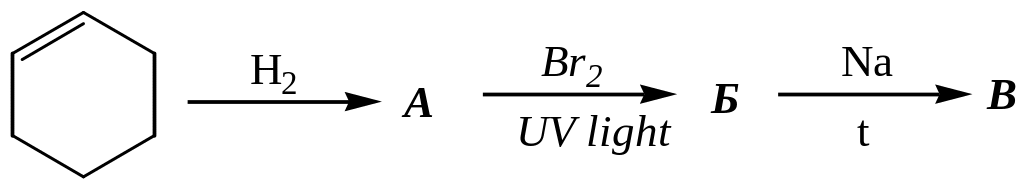 а) hexane,
2-bromohexane,
5,6-dimethyloctane;;
b) ethane,
bromoethane,
butane;
с) butane,
bromoethane,
butane;
d) cyklohexane,
bromocyklohexane,
bicyklohexane
а) hexane,
2-bromohexane,
5,6-dimethyloctane;;
b) ethane,
bromoethane,
butane;
с) butane,
bromoethane,
butane;
d) cyklohexane,
bromocyklohexane,
bicyklohexane
What is the structure of hydrocarbon С8Н18 that has branched chain, if it was received by electrolysis of water solution of salts of carboxylic acid, that creates tetramethylmethane while fusion with the lye? Write the equations. а) 2-methylheptane; b)2,5-dimethylhexane; с) 2,2,3,3-tetramethylbutane; d) 3,4-dimethylhexane;
What is the structure of carboxylic acid, if while fusion its salt with the lye isobutane is created. While electrolysis of water solution of its salt 2,5-dimethylhexane is created. Write the equations. а) isopentanoic acid; b) isobutanoic; с) neopentanoic; d) butanoic.
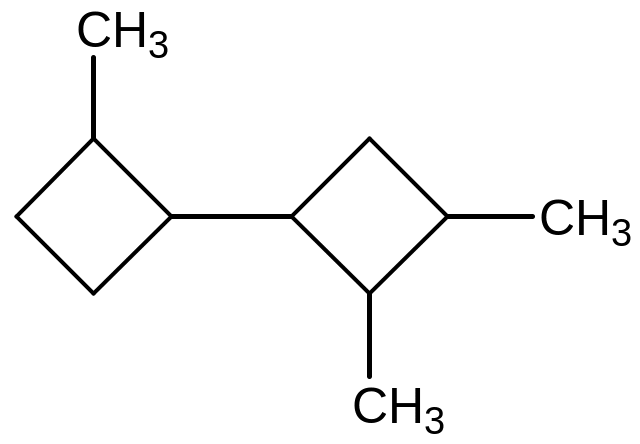 Name
the
compound
а) 2,2’,3-
trimethylbicyklobutane;
b) 2,3’,4’- trimethylbicyklobutane;
с) 2,2,3- trimethylbicyklobutane;
d) 2,3,4- trimethylbicyklobutane;
Name
the
compound
а) 2,2’,3-
trimethylbicyklobutane;
b) 2,3’,4’- trimethylbicyklobutane;
с) 2,2,3- trimethylbicyklobutane;
d) 2,3,4- trimethylbicyklobutane;
 Name
the
compound
а) 2-ethyl-3,9-dimethylbicyklo
[4,3,0]nonan; b)
1,7 -dimethyl-8-ethylbicyklo
[6,5,2] nonan;
с) 1-ethyl
-2,8- dimethylbicyklo
[5,4,1] nonan;
d) 3,9-dimethyl-2-
ethylbicyklo
[4,3,0] nonan
Name
the
compound
а) 2-ethyl-3,9-dimethylbicyklo
[4,3,0]nonan; b)
1,7 -dimethyl-8-ethylbicyklo
[6,5,2] nonan;
с) 1-ethyl
-2,8- dimethylbicyklo
[5,4,1] nonan;
d) 3,9-dimethyl-2-
ethylbicyklo
[4,3,0] nonan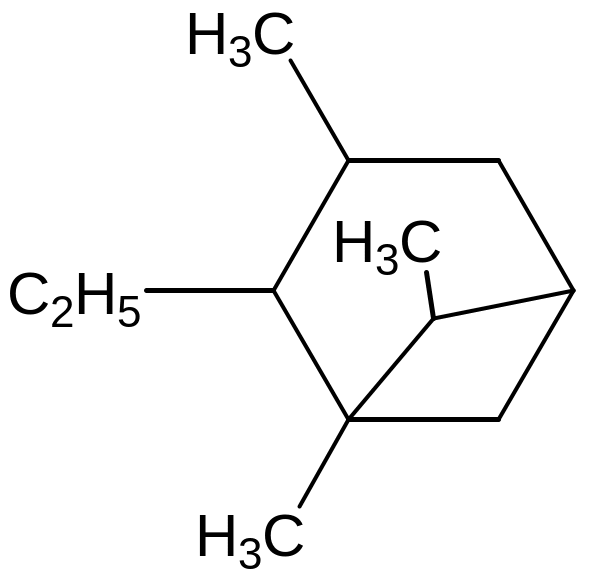 Name
the compound
Name
the compound
а) 2-ethyl-1,3,6- trimethylbicyklo [3,1,1] hexane;
b) 1,3,7-trimethyl-2-ethylbicyklo [4,4,3] hexane;
с) 1-ethyl-2,6,7- trimethylbicyklo [2,2,1] hexane;
d) 2-ethyl-1,3,6- trimethylbicyklo [5,3,3] hexane;
