
- •1 Alkanes
- •1.1 Nomenclature of Alkanes
- •1.2 Physical Properties of Alkanes
- •1.3 Preparation of Alkanes
- •Industrially Preparation
- •1.4 Reactions of Alkanes
- •3. Chain termination
- •1.5 Cycloalkanes
- •1.6 Nomenclature of Cycloalkanes
- •1.7 Isomerization of Cycloalkanes
- •1.8 Reactions of Cycloalkanes
- •2 Alkenes
- •2.1 Nomenclature of Alkenes
- •2.2 Physical Properties of Alkenes
- •2.3 Preparation of Alkenes
- •2.4 Reactions of Alkenes
1.3 Preparation of Alkanes
Petroleum Refining
1st step: fractional distillation
Petroleum is separated into different fractions in the fractionating tower
Each fraction is a simple mixture and has specific uses
Cracking
Cracking is the process of breaking down of large alkane molecules in the heavier fractions of petroleum into lighter fractions of smaller molecules in the absence of air
e.g. C11H24 —® C9H20 + CH2=CH2
C14H30 —® C8H18 + 2CH2=CH2 + 2C + 2H2
Catalytic cracking :
in the presence of catalyst, smaller and highly branched hydrocarbons formed
Thermal cracking :
in the absence of catalyst, unbranched chains formed
Reforming
Reforming is a process in which straight-chain alkanes are heated under pressure in the presence of a platinum catalyst. The chains break up and reform to give branched-chain molecules.
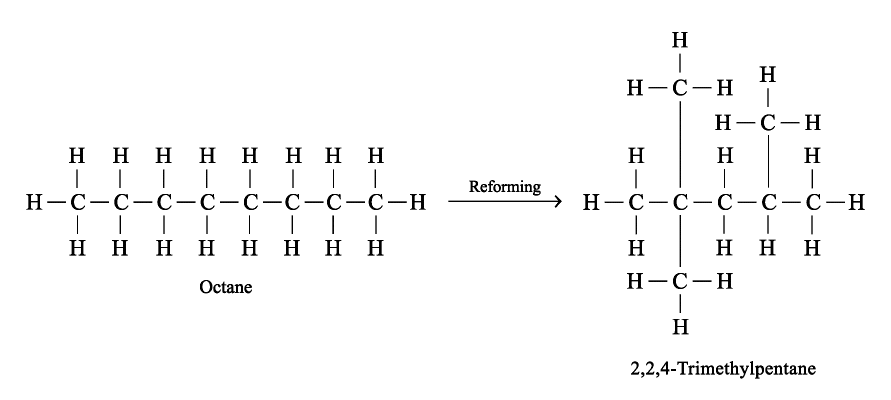
Industrially Preparation
Fischer-Tropsch’s synthes
![]()
Alkynes and alkenes catalytic hydrogenization
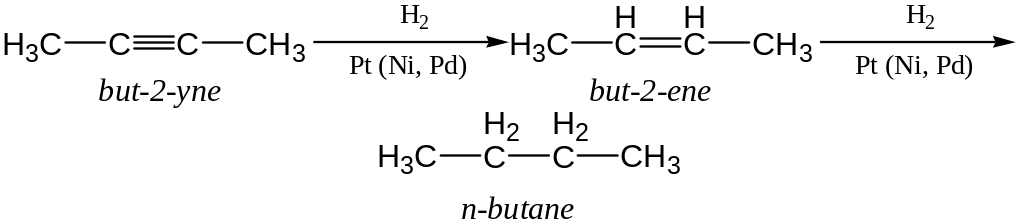
Laboratory Preparation
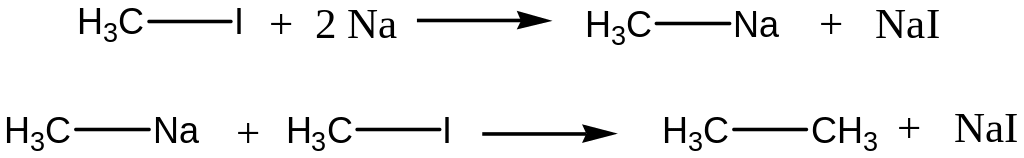
Halogenalkanes and metallic Na interaction (Wurtz’s reaction)
![]()
Reaction Mechanism
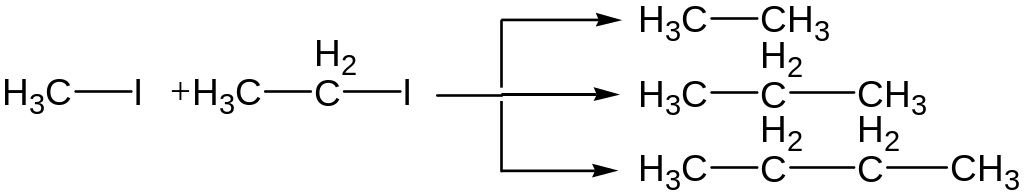
In case reaction with two different halogenalkanes a mixture of products is formed
Fusion of salts of carboxylic acids with lyes

Metalloorganic compounds and water interaction
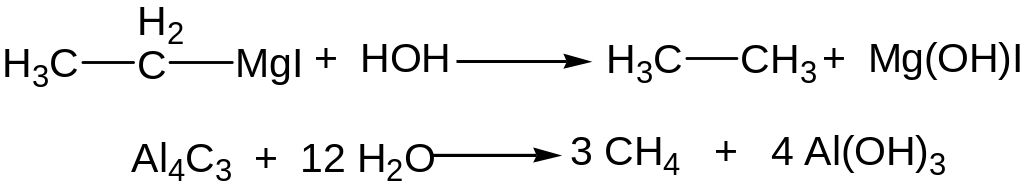
Electrolysis of water solutions of salts of carboxylic acids
![]()
Anodic reaction mechanism
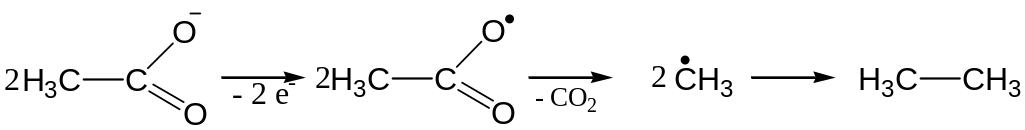
1.4 Reactions of Alkanes
Combustion
Complete combustion :
Alkanes react with sufficient oxygen to give carbon dioxide and water through a complex series of reaction with the release of a large amount of energy.
General formula:
![]()
Methane: main component of natural gas and domestic gas
Butane: component of bottle gas
Combustion reaction: free radical reaction. The energy released during combustion dominates a large part of world’s transport, industry and domestic heating.
In the limited supply of oxygen, alkanes burn to give CO(g) and carbon particles
![]()
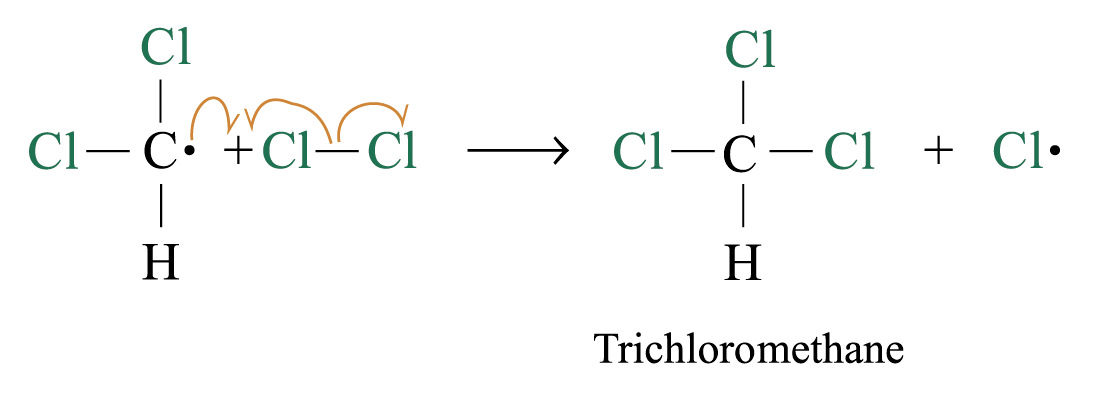
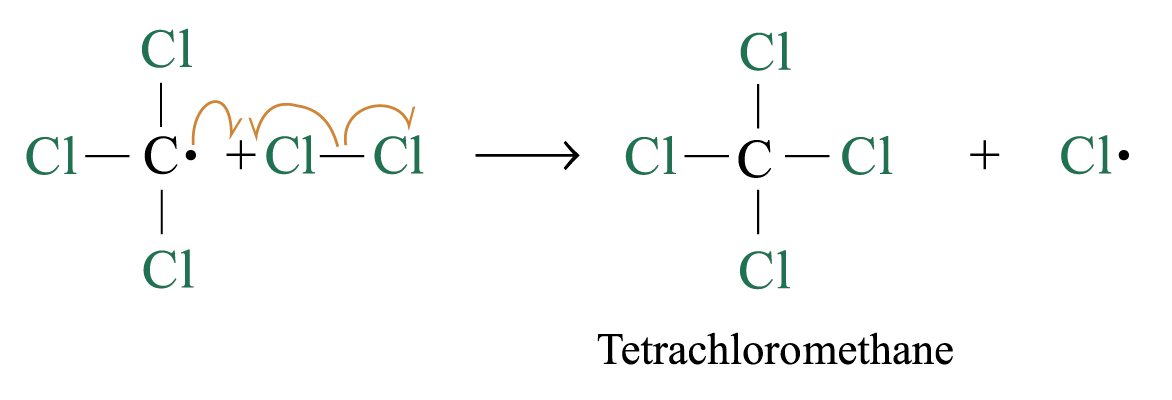
Chlorination
Methane reacts with chlorine under diffuse sunlight or heating but not in dark
A mixture of products (CH3Cl, CH2Cl2, CHCl3, CCl4) is formed with the replacement of hydrogen by one or more chlorine atom
![]()
![]()
![]()
![]()
If methane is in excess, CH3Cl is major product. If chlorine is in excess, CCl4 is major product
Reaction Mechanism: Free Radical Substitution Reaction
1. Chain initiation
homolytic fission of chlorine molecules by heat or light into two chlorine radicals
Step 1:
![]()
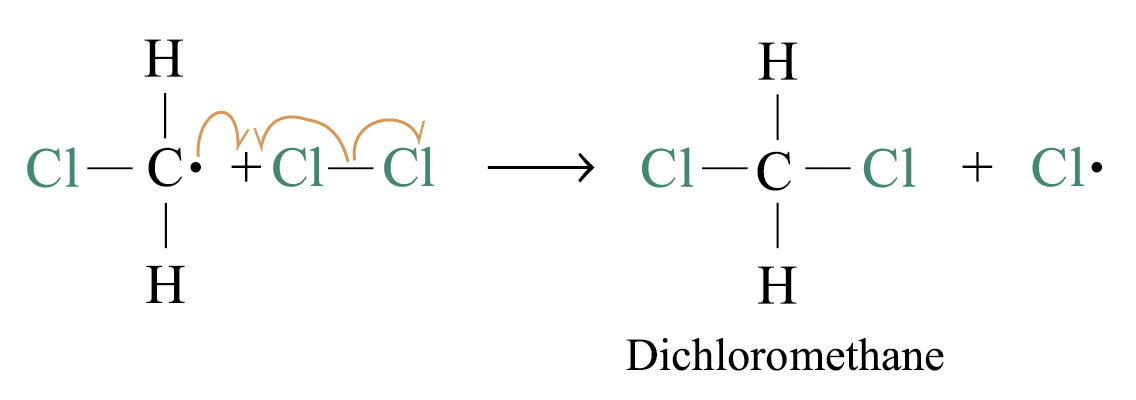
2. Chain propagation
Step 2:

Step 3:
![]()
steps 2 and 3 repeat hundreds or thousands of time due to formation of the reactive intermediate in each step Þ chain reaction
Further substitution occurs:
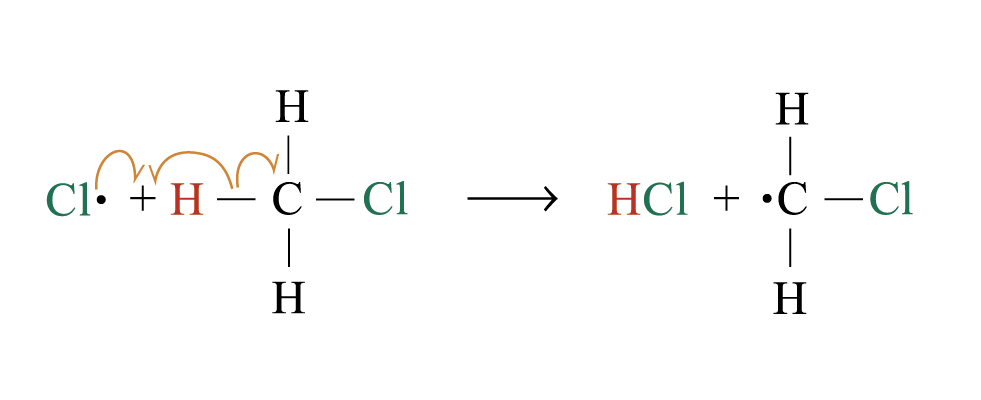
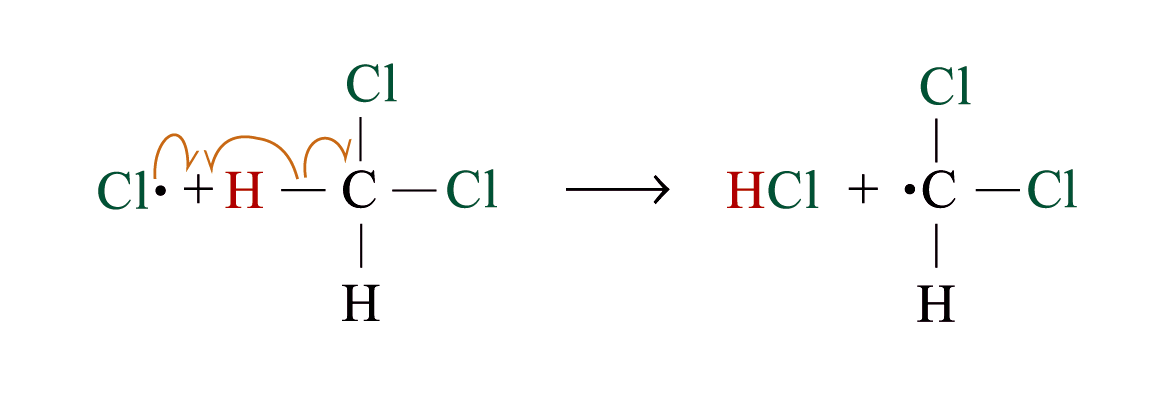
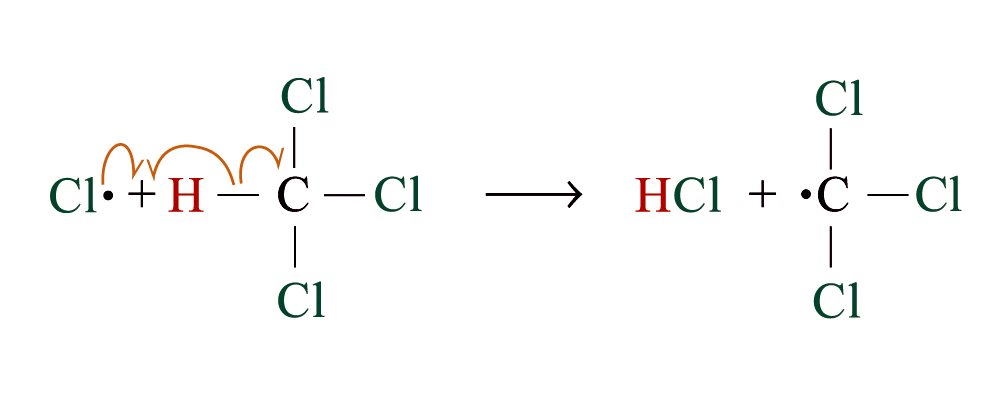
3. Chain termination
two free radicals combine to form a neutral molecule: the chain reaction is terminated
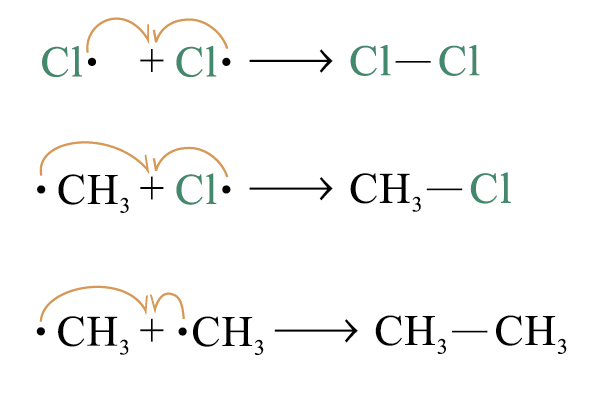
In cace branched-chain acyclic hydrocarbons monochlorination a mixture of products is formed with the replacement of tertiary, secondary or primary hydrogen by one chlorine atom

Because tert-carboradical is the most stable
Nitration (Konovaloff’s reaction)
![]()
Reaction Mechanism: Free Radical Substitution Reaction
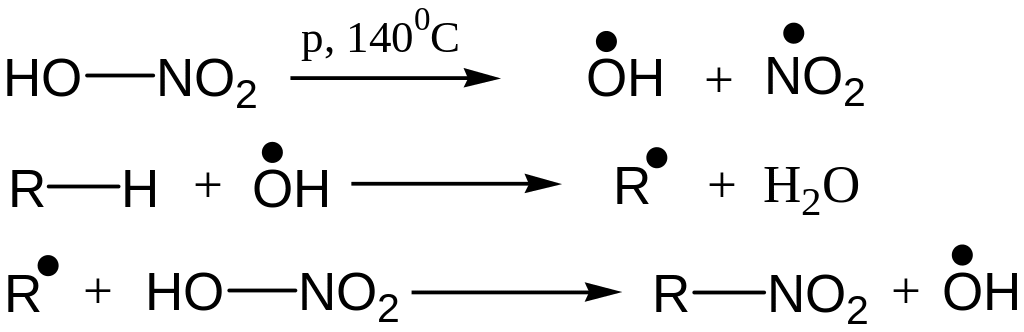
Like Chlorination, tert-hydrogen is replaced easiest
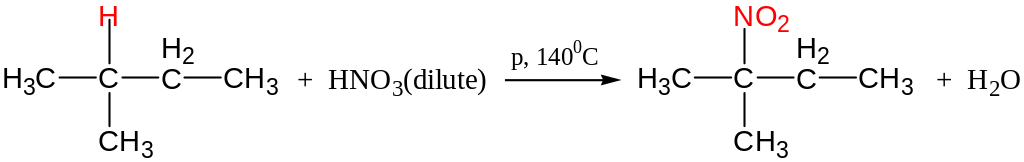
Sulfohlorination
![]()
Reaction Mechanism: Free Radical Substitution Reaction
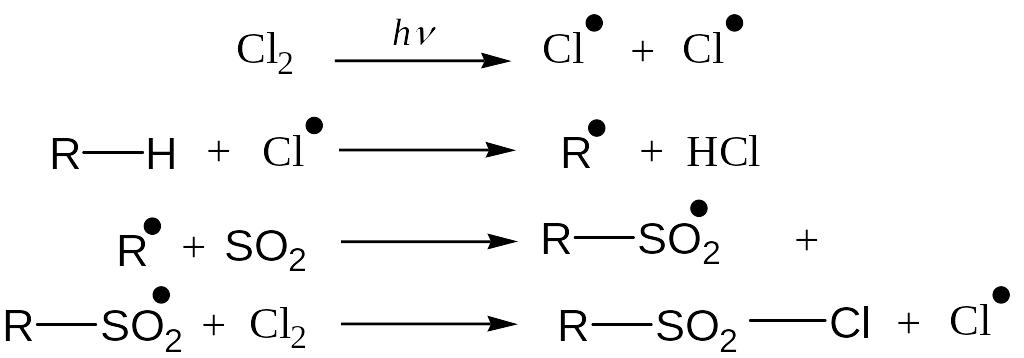
Unlike chlorination and nitration, tert-hydrogen is not replaced due to steric hindrance. In result of reaction a mixture of prim- and sec-sulfochlorides is formed.
