
- •Protein phosphorylation as a switch in cellular functioning
- •Cyclic AMP and the amplification of signals
- •Protein kinase A
- •Protein kinase A and the regulation of transcription
- •Activation of the CREB transcription factor
- •Attenuation of the cAMP response elements by dephosphorylation
- •Protein kinase A and the activation of ERK
- •Actions of cAMP not mediated by PKA
- •Regulation of ion channels by cyclic nucleotides
- •Epac, a guanine nucleotide exchange factor directly activated by cAMP
- •Protein kinase C
- •Discovery of a phosphorylating activity independent cAMP
- •The protein kinase C family
- •Structural domains and activation of protein kinase C
- •The C1–C4 regions
- •Activation of protein kinase C
- •Multiple sources of diacylglycerol and other lipids activate protein kinase C
- •Differential localization of PKC isoforms
- •Different types of PKC-binding proteins
- •Holding back the PKC response
- •A matter of life or death: PKC signalling complexes in the evasion of the fly-swat
- •Phorbol ester and inflammation
- •References

Phosphorylation and Dephosphorylation: Protein Kinases A and C
recruits atypical PKCs into TNFand interleukin-1 (IL-1) receptor complexes and contributes to the activation of the NF- B pathway. p62 also links PKC with the potassium channel subunit Kv 2 and to the growth factor receptor bound Grb14. PKC phosphorylates RelA (a component of NF- B complex) in the nuclear localization signal motif, so promoting its transcriptional activity.80 Par-6, to which we return later (page 585), links atypical PKC to the Rho family of GTPases, Cdc42, and Rac1 involved in the regulation of cell polarity.81
The primary function of all these anchoring proteins is to position individual PKCs into the precise locations in which they can respond to specific receptor-mediated activating signals and address their substrates. Given the promiscuity of PKC in test-tube kinase assays, the anchoring proteins may act to prevent inappropriate phosphorylation events in the cellular environment. A similar theme emerges with the serine/threonine protein phosphatases (see Chapter 21).
Holding back the PKC response
Diacylglycerol kinases
Phosphorylation of DAG to form phosphatidic acid provides a means to deactivate PKC. The family of diacylglycerol kinases (DAG kinases, 9 members) is subdivided on the basis of either substrate specificity or the presence of specific domains. They all have C1 or extended C1 domains (15 extra amino acids) and they all have catalytic and accessory domains (see also PI 3-kinase, in Chapter 18). Despite the presence of a C1 domain, only DAG kinases andinteract with phorbol ester. The type I DAG kinases (comprising , , and ) are characterized by two Ca2 -binding EF-hands. Type II kinases (comprisingand ) are characterized by PHand SAM domains (sterile motif). The SAM domain allows binding to the endoplasmic reticulum and plays a role in vesicle trafficking. Type III (only ) is without recognizable additional domains. Type IV kinase (comprising and ) have a MARCKS-homology sequence, which acts as a nuclear localization signal. Type V (only ) possesses three
C1 domains.
Although conclusive evidence is still lacking, the DAG kinases are believed to be quiescent until their activity is required. This is achieved by recruitment to the membrane by binding cofactors and phosphorylation, by for instance, activated PKC.82
Phosphatidic acid
Although DAG kinase removes DAG and thus terminates membrane localization of PKC, it does not necessarily stop membrane signalling, because the product phosphatidic acid (phosphatidate) itself has numerous effects. It stimulates DNA synthesis, it helps recruitment of Raf to the membrane, and it modifies the activity of RasGAP and of PKC (which does not respond to DAG).83
263
Signal Transduction
Down-regulation by phorbol esters
Prolonged activation by phorbol esters results in a loss of PKC activity. This is the consequence of both dephosphorylation of critical residues and
degradation of the protein. Prior treatment of cells with phorbol ester, leading to depletion of a some PKC isoforms, has been extensively applied in studies that test for a role for PKC in physiological processes.
A matter of life or death: PKC signalling complexes in the evasion of the fly-swat
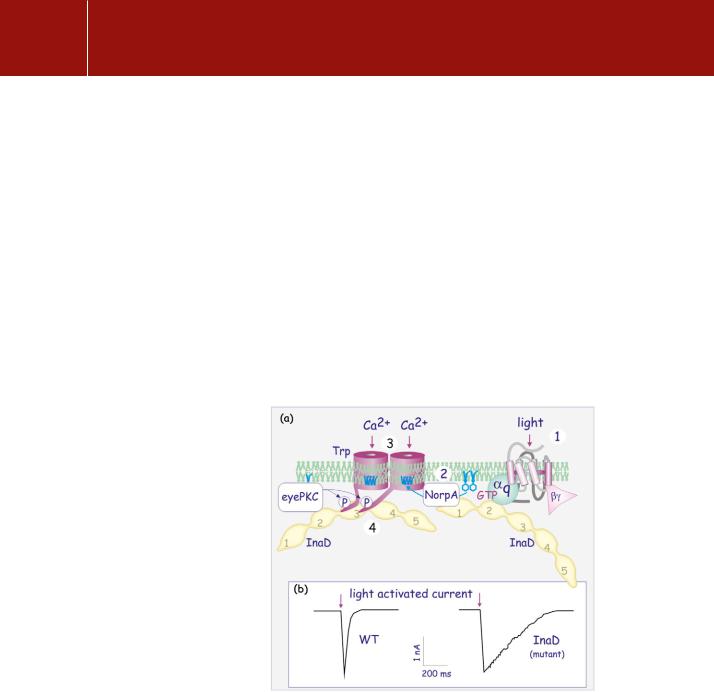
Anyone who has tried unsuccessfully to swat a fly must realize that the speed of their visual response is faster by far than ours. More than this, the sensitivity of their photosensors and the capacity to adapt far outstrips that of any vertebrate. The phototransduction cascade in Drosophila (see Figure 6.13, page 175) reveals how scaffold proteins operate in signal transduction through PKC (Figure 9.13). Photoactivation of rhodopsin causes the opening
FIG 9.13 InaD scaffold protein and signalling complexes.
(a) The formation of a complex that operates in Drosophila phototransduction. Two copies of InaD gather a number of signalling molecules to form a signalling circuit. Light falls on rhodopsin (1) which activates G q, which acts on NorpA, a PLC (2). This causes the generation of DAG, which opens a Trp Ca2 channel (3), causing membrane
depolarization , (see page 210). This rapid event is followed by an almost instantaneous desensitization of Trp due to phosphorylation by eye-PKC (InaC). PDZ domains are numbered 1–5. (b) A loss of InaD causes a retardation of the return to the resting state and hence a loss of visual resolution.
Adapted from Xu et al.84
264
Phosphorylation and Dephosphorylation: Protein Kinases A and C
of the light-sensitive cation influx channels Trp and TrpL. This rapid response results in a very transient depolarization of the photoreceptor cells. Most of the proteins that operate in the phototransduction cascade associate as a single supramolecular signalling complex. The key component in this complex is InaD, which has multiple PDZ domains. InaD together with InaC, a protein kinase C, emerged in genetic screens for mutations in Drosophila visual transduction.
InaD, present in the microvillar compartment of the photoreceptor (the rhabdomere: see Figure 12.9, page 329) is closely associated with several components including a -type PLC (gene product of NorpA), the cation influx channels (Trp and TrpL), InaC, the eye-specific Drosophila variant of PKC (eye-PKC), and the photosensor rhodopsin (product of the ninaE gene).84 They are attached through the PDZ domains of InaD. PDZ-1 attaches PLC (NorpA), PDZ-2 attaches eye-PKC and q, and PDZ-3 attaches to Trp (Figure 9.13). At least two InaDs are required to gather all the signal transduction components involved, linking the rhodopsin to the ion channel. This supramolecular complex ensures both the rapid opening of the Ca2 channel and then its almost instantaneous desensitization due to phosphorylation of Trp by InaC.85 PP1/PP2A-like phosphatases, preferentially expressed in photoreceptor cells, act as the reset button, and so allowing a new cycle of excitation to follow.86
Disruption of any one of these interacting components alters the photoresponse. In InaD mutants, the various components of the signalling complex, such as the Ca2 -channel, typePLC, and eye-PKC, are no longer exclusively located in the rhabdomeres, though rhodopsin and the Ca2 -channel (intrinsic membrane proteins) of course remain. Photoreceptor cells in flies that have mutations at either InaC or InaD desensitize sluggishly, remaining depolarized. Such flies have problems distinguishing light of different intensities and flash duration.87
The macromolecular assembly avoids random collisions of individual components of the signalling pathway and ensures high precision.
Phorbol ester and inflammation
The phorbol ester PMA is a skin irritant and inflammatory agent. It induces prompt remodelling of the vasculature, resulting in oedema,88 and the response is enhanced by the induced secretion of histamine from mast cells and blood-borne basophils (Figure 9.14).89,90 It also activates adhesion molecules present on the surface of leukocytes, causing them to bind and then to migrate through the vascular endothelial layer into the tissues underlying the skin.91 In the tissues, PMA potentiates antigen-mediated stimulation of T lymphocytes, enhancing the production of the cytokine interleukin-2 (IL-2), essential for the induction of clonal expansion (see Chapter 17).92 This is mediated through activation of PKC and the
The symptoms of inflammation were summarized for all time by the 1st century Roman encyclopaedist Celcius as tumor, calor, rubor, and dolor (swelling, warmth, redness, and pain). Inflammation, now recognized as a type of non-specific immune response, represents the
basic means by which the body reacts to infection, irritation or injury.
265
