
Карцев В.Г.Избранные методы с-за и модифик. гетероциклов т.1 , 2003
.pdf
However, the reactivity of N,S-acetals 1f–h without any activating group in aniline were found to be sluggish with Vilsmeier reagent requiring prolonged heating at higher temperature to afford the respective quinolines in poor yields (entries 6–8 ). On the other hand, N,S-acetal 1i derived from α-naphthylamine was efficiently transformed into condensed 2-methylthio-3-benzoyl-benzo[h]quinoline 2i in nearly quantitative yield on treatment with Vilsmeier reagent under identical conditions as for 1a–e (Table 1, entry 9). The N,S-acetals 1a–c, i also underwent rapid cyclization with Vilsmeier reagent derived from N,N-dimethylacetamide to furnish respective 2-methylthio-3-benzoyl- 4-methylquinolines 3a–c (Scheme 2) and corresponding benzo[h]quinoline derivative 3i in excellent yields (entries 10–13).
Similarly, corresponding N,S-acetals 1k–l obtained from pyruvaldehyde dimethylacetal afforded respective 3-(bismethoxy)acetylquinolines 2k–l in reasonable yields (Table 1, entries 14–15).
Table 1
|
|
N,S-acetals 1 |
|
|
|
Method |
Quinolines 2, 3 |
|
|
|
Yield, |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
1 |
|
|
|
|
Ar |
a |
|
|
|
|
|
Ar |
81 |
|
|||||
2 |
|
|
|
|
|
|
O |
a |
|
|
|
|
|
|
|
O |
95 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
MeO |
N |
SMe |
|
MeO |
N |
SMe |
|
|
|||||||||
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1a Ar = Ph |
|
|
|
|
|
|
2a Ar = Ph |
|
|
|
|
|
|
|
|||
|
|
1b Ar = 2-BrC6H4 |
|
|
|
|
2b Ar = 2-BrC6H4 |
|
|
|
|
|
|||||||
3 |
|
|
|
|
Ar |
a |
|
|
|
|
|
|
Ar |
98 |
|
||||
4 |
MeO |
|
|
|
|
O |
a |
MeO |
|
|
|
|
O |
95 |
|
||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
MeO |
N |
|
SMe |
|
MeO |
N |
|
SMe |
|
|
|||||||
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1c Ar = Ph |
|
|
|
|
|
|
2c Ar = Ph |
|
|
|
|
|
|
|
|||
|
|
1d Ar = 2-BrC6H4 |
|
|
|
|
2d Ar = 2-BrC6H4 |
|
|
|
|
|
|||||||
5 |
OMe |
Ph |
|
|
|
a |
OMe |
Ph |
|
|
|
90 |
|
||||||
|
|
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
N |
SMe |
|
|
|
N |
SMe |
|
|
||||||||
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
OMe H |
|
|
|
|
|
|
|
|
OMe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
1e |
|
|
|
|
|
|
|
|
2e |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Генеральный спонсор и организатор – InterBioScreen Ltd. |
|
|
|
|
|
247 |
||||||||||||
Table 1. Continued
6 |
|
Ph |
b |
|
Y |
Ph |
30 |
|
|
O |
|
|
|
O |
10 |
|
|
|
|
|
|
||
F |
N |
SMe |
|
X |
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1f |
|
|
2fa X = F, Y = H |
|
||
|
|
|
2fb X = H, Y = F |
|
|||
|
|
|
|
|
|||
7 |
|
Ph |
b |
|
|
Ph |
25 |
|
|
O |
|
|
|
O |
|
|
N |
SMe |
|
|
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1g |
|
|
|
2g |
|
|
8 |
|
Ph |
b |
|
|
Ph |
30 |
Cl |
|
O |
|
Cl |
|
O |
|
|
|
|
|
|
|
||
|
N |
SMe |
|
|
N |
Cl |
|
|
H |
|
|
|
|
|
|
|
1h |
|
|
|
2h |
|
|
9 |
|
Ph |
a |
|
|
Ph |
95 |
|
|
O |
|
|
|
O |
|
|
N |
SMe |
|
|
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1i |
|
|
|
2i |
|
|
10 |
|
Ar |
c |
|
|
Ar |
80 |
11 |
|
O |
c |
|
|
O |
95 |
|
|
|
|
|
|
||
MeO |
N |
SMe |
|
MeO |
N |
SMe |
|
|
H |
|
|
|
|
|
|
1a Ar = Ph |
|
|
3a Ar = Ph |
|
|
||
1b Ar = 2-BrC6H4 |
|
3b Ar = 2-BrC6H4 |
|
||||
248 |
Устные доклады |
Table 1. Continued
12 |
|
Ph |
c |
|
|
Ph |
98 |
MeO |
|
O |
|
MeO |
|
O |
|
|
|
|
|
|
|
||
MeO |
N |
SMe |
|
MeO |
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1c |
|
|
|
3c |
|
|
13 |
|
Ph |
c |
|
|
Ph |
95 |
|
|
O |
|
|
|
O |
|
|
N |
SMe |
|
|
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1i |
|
|
|
3i |
|
|
14 |
MeO |
OMe |
a |
|
MeO |
OMe |
51 |
|
|
O |
|
|
|
O |
|
MeO |
N |
SMe |
|
MeO |
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1k |
|
|
|
2k |
|
|
15 |
MeO |
OMe |
a |
|
MeO |
OMe |
54 |
MeO |
|
O |
|
MeO |
|
O |
|
|
|
|
|
|
|
||
MeO |
N |
SMe |
|
MeO |
N |
SMe |
|
|
H |
|
|
|
|
|
|
|
1l |
|
|
|
2l |
|
|
a: HCONMe2, POCl3, ∆, 80°C; |
|
|
|
|
|
||
b: HCONMe2, POCl3, Cl2CHCHCl2, ∆, 80°C; |
|
|
|
|
|||
c: MeCONMe2, POCl3, ∆, 80°C |
|
|
|
|
|
||
Генеральный спонсор и организатор – InterBioScreen Ltd. |
|
|
249 |
||||
Scheme 2
|
O |
R'' |
O |
R |
R' a or b or c |
R |
R' |
|
|
||
|
N SMe |
N |
SMe |
|
H |
|
|
|
1 |
2, 3 |
|
a: HCONMe2, POCl3, ∆, 80°C
b: HCONMe2, POCl3, Cl2CHCHCl2, ∆, 80°C
c: MeCONMe2, POCl3, ∆, 80°C
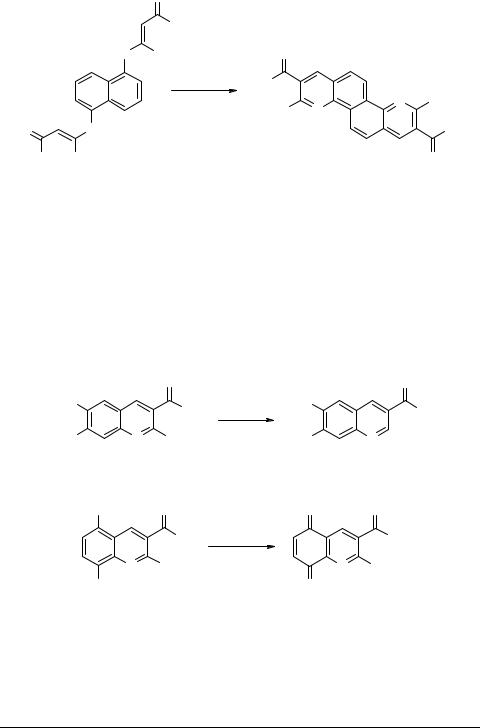
The validity of this new quinoline synthesis was further evaluated by performing the Vilsmeier reaction on bisketene-N,S-acetals 4, 6 and 8 with a view to synthesize planar tricyclic and tetracyclic heterocyclo fused quinolines (Scheme 3). Thus the biske- tene-N,S-acetal 4 from m-phenylenediamine gave corresponding angularly fused azaphenanthridine 5 in 48% yield, while corresponding linearly fused bisquinoline could not be isolated from the reaction mixture. Bisketene-N,S-acetal 6 from o-phenylenediamine however required drastic conditions (POCl3/DMA/TCE, 130°C), and the product isolated after workup was characterized as 2-methythio-9-hydroxy-4,7-diphenylphenanthroline 7 on the basis of its spectral and analytical data. In the absence of any activation at the site of cyclization with Vilsmeier reagent, intramolecular Combe's type cyclization of the enaminone functionality is the preferred cyclization mode to yield the observed product 7 [4]. Bisketene-N,S-acetal 8 from 1,5-diaminonaphthalene underwent smooth cyclization with Vilsmeier reagent to afford 2,8-bis(benzoyl)-3,9-bis(methylthio)quinolino[8,7-b]- quinoline 9 in 60% yield (Scheme 3).
|
|
|
|
|
|
|
Scheme 3 |
O |
|
O |
|
|
|
|
|
Ph |
|
Ph |
DMF, POCl3 |
N |
|
|
O |
|
MeS |
|
|
|
|||
|
|
|
|
|
N |
Ph |
|
MeS N |
N |
SMe |
|
|
|
||
H |
H |
|
|
O |
|
|
SMe |
|
4 |
|
|
Ph |
5 |
(48%) |
|
|
|
|
|
|
O |
|
|
|
|
O |
Ph |
Ph |
|
|
|
|
DMF, POCl3 |
|
||
|
|
|
|
|
|
|
|
Ph |
|
N |
N |
|
Ph |
TCE, 130°C |
N N |
|
|
|
|||||
|
MeS |
H |
H |
SMe |
|
HO |
SMe |
|
|
6 |
|
|
|
|
7 (60%) |
250 |
Устные доклады |
|
|
O |
|
|
|
|
|
Ph |
|
|
|
|
|
HN SMe |
O |
|
|
|
|
HCONMe2 |
Ph |
|
|
|
|
POCl3, ∆ |
MeS N |
|
N SMe |
|
|
|
|
|
|
O |
NH |
|
|
|
Ph |
Ph |
SMe |
8 |
|
9 (60%) |
O |
|
|
|
|
With a variety of multifunctional, substituted and fused methylthioquinolines in hand, we further explored the possible transformations of these functionalities to afford new quinolines. Thus, the 2-methylthio group in quinolines 2a and 2c could be reductively removed with Raney Ni to afford 2-unsubstituted quinolines 10a and 10c in good yields. Similarly, corresponding 2-methylthio-3-benzoyl-5,8-dimethoxy quinoline 1e was subjected to oxidative demethylation in the presence of aqueous NBS/H2SO4 to afford quino- line-5,8-quinone 11 in high yield [5] (Scheme 4).
Scheme 4
|
|
O |
|
|
O |
X |
|
Ph |
Raney Ni |
X |
Ph |
|
|
|
|||
MeO |
N |
SMe |
EtOH, ∆ |
MeO |
N |
|
|||||
|
2a X = H |
|
|
10a X = H (70%) |
|
|
2c X = OMe |
|
|
10c X = OMe (75%) |
|
|
OMe |
O |
|
O |
O |
|
|
Ph |
NBS, H2SO4 |
|
Ph |
|
N |
SMe |
THF, H2O |
N |
SMe |
|
|
||||
|
OMe |
|
|
O |
|
|
2e |
|
|
11 (75%) |
|
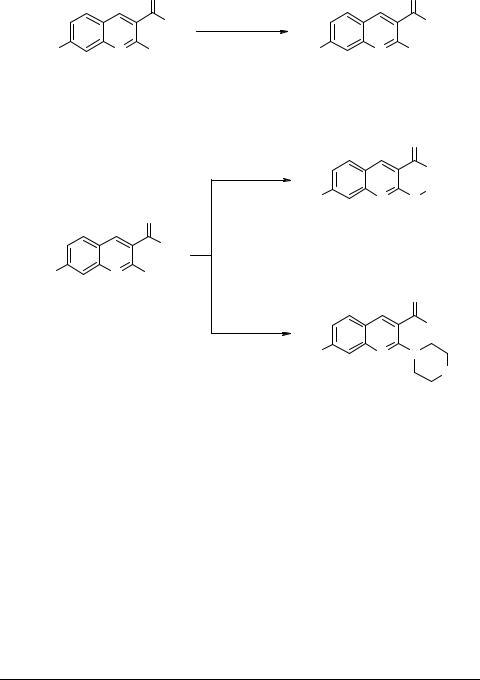
The 2-methylthio group in quinoline 2a could be oxidized with m-chloroperbenzoic acid to afford corresponding 2-(methylsulfonyl)quinoline 12a in 75% yield. The 2-me- thylsulfonyl group in 12a could be easily displaced by primary and secondary amines under varying conditions to afford corresponding 2-alkyl/aryl amino quinolines 13–16 in high yields (Scheme 5).
Генеральный спонсор и организатор – InterBioScreen Ltd. |
251 |
Scheme 5
O
Ph
MeO |
N SMe |
2a
|
O |
|
Ph |
MeO |
N SO2Me |
|
12a |
|
|
|
O |
|
MCPBA, CH2Cl2 |
|
|
|
Ph |
0°C to r.t. |
MeO |
N |
SO2Me |
|
|
||||
|
|
12a (75%) |
|
|
RNH2, THF, ∆ |
|
|
O |
|
|
|
|
Ph |
|
or |
|
|
|
|
PhNH2, MW |
MeO |
N |
N |
R |
|
|
|||
|
|
|
H |
|
|
|
13 R = Bu (86%) |
||
|
|
14 R = Bn (93%) |
||
|
|
15 R = Ph (77%) |
||
|
|
|
O |
|
morpholine |
|
|
|
Ph |
∆ |
MeO |
N |
N |
|
|
|
|||
O
16 (70%)
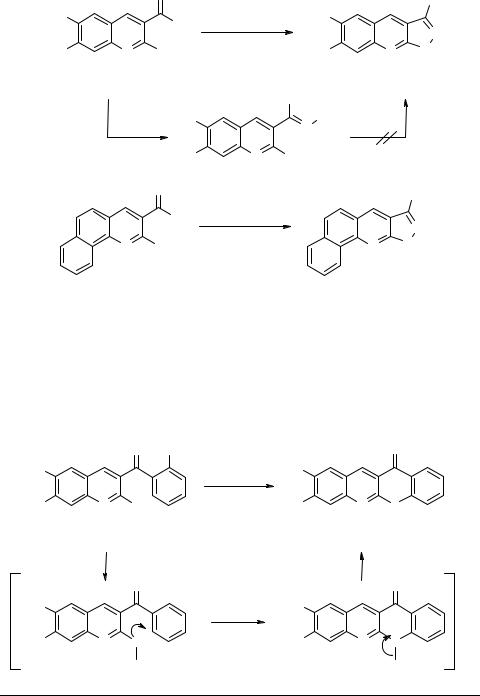
Quinolines 2a, c, i were next subjected to annelation reaction with hydrazine hydrate with a view to synthesize pyrazolo[3,4-b]quinolines 17a, c, i which are known to exhibit various biological activities such as antiviral, antimalarial and lowering of serum cholesterol [6]. Recently, 3-phenylpyrazolo[3,4-b]quinoline-4-one has been shown to display pH dependent fluorescent properties for extreme pH measurement [7]. Thus, reaction of 2c with hydrazine hydrate afforded hydrazone 18c in nearly quantitative yield. Hydrazone 18c failed to undergo cyclization to pyrazolo[3,4-b]quinoline 17c even after prolonged refluxing, which is presumably due to adoption of unfavorable E-configuration. However, pyrazoloquinolines 17a, c could be obtained directly from corresponding quinolines 2a, c in high yields by reacting them with hydrazine hydrate under microwave irradiation conditions [6]. Similarly, synthesis of corresponding tetracyclo-10H-benzo- [h]pyrazolo[3,4-b]quinoline 17i could be achieved from benzo[h]quinoline precursor 2i following a similar procedure (Scheme 6). Many of these pyrazolo-fused benzo[h]quinolines are shown to bind with DNA inhibiting topoisomerase I activity [8].
252 |
Устные доклады |
Scheme 6
|
|
O |
|
|
|
|
|
Ph |
X |
|
|
|
|
|
X |
|
|
|
Ph |
N2H4·H2O, PTSA |
|
|
|
|||
|
|
|
|
|
N |
|||
|
|
|
|
MW, 3 min |
|
|
|
|
MeO |
N |
SMe |
|
MeO |
N |
N |
||
|
|
|||||||
|
2a X = H |
|
|
|
|
|
|
H |
|
|
|
|
|
|
17a X = H (71%) |
||
|
2c X = OMe |
|
|
Ph |
|
|
17c X = OMe (74%) |
|
|
|
|
|
|
|
|
|
|
|
N2H4·H2O |
X |
|
N |
NH2 |
∆ |
|
|
|
|
|
|
|
||||
|
EtOH, ∆ MeO |
N SMe |
|
|
|
|||
|
|
O |
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
N2H4·H2O, PTSA |
|
|
|
N |
|
|
|
|
MW, 3 min |
|
|
|
|
|
N |
SMe |
|
|
|
N |
N |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
H |
|
2i |
|
|
|
|
|
17i (85%) |
|
Finally, further scope of the functional group manipulation in these quinolines was demonstrated by performing radical cyclization of 3-(o-bromobenzoyl)quinolines 2b by treatment with Bu3SnH/AIBN (Scheme 7). The product formed in nearly quantitative yield was characterized as the novel benzothiopyrano[2,3-b]quinoline derivative 18b on the basis of its spectral and analytical data. Similarly, 3-(o-bromobenzoyl)-6,7-di- methoxyquinoline 2d afforded tetracyclic benzothiopyrano fused quinoline 18d in 98% yield .The probable mechanistic pathway for the formation of 18b and 18d is shown in Scheme 7.
Scheme 7
|
|
O Br |
|
|
O |
X |
|
|
X |
|
|
|
|
|
TBTH, AIBN |
|
|
MeO |
N |
SMe |
∆, toluene MeO |
N |
S |
|
2b X = H |
|
18b X = H (98%) |
||
|
2d X = OMe |
18d X = OMe (98%) |
|||
|
|
|
|
−Me· |
|
|
|
O |
|
|
O |
X |
|
|
X |
|
|
MeO |
N |
S · |
MeO |
N |
· |
S |
|||||
|
19 |
|
|
20 |
|
Генеральный спонсор и организатор – InterBioScreen Ltd. |
|
253 |
|||

Initially formed o-benzoyl radical 19 undergoes radical translocation [9] by attack on the methylthio group to give radical intermediate 20 which, on loss of methyl radical, affords benzothiopyrano quinolines 18b and 18d in excellent yields.
Conclusions
We have developed a simple, highly efficient and regioselective synthesis of functionalized 2-methylthio-3-substituted quinolines through Vilsmeier cyclization of a variety of α-oxoketene-N,S-acetals. The 2-methylthio functionality in these quinolines has been further manipulated to afford either 2-alkyl/arylamino quinolines, pyrazolo[3,4-b]quino- lines and benzothiopyrano[b]quinolines through ring annelation with hydrazine hydrate or via radical cyclization.
References
1.(a) Junjappa H., Ila H., Asokan C.V., Tetrahedron 1990 46 5423, Tetrahedron Report No. 278; (b) Tominaga Y., J. Heterocycl. Chem. 1989 26 1167; (c) Ila H., Junjappa H., Mohanta P.K., in Progress in Heterocyclic Chemistry, Gribble G.W., Gilchrist T.L., Eds., Oxford: Pergamon Press, 2001, vol. 13, p. 1.
2.Mahato P.K., Venkatesh C., Syam Kumar U.K., Ila H., Junjappa H., J. Org. Chem. 2003 69 (in press).
3.Singh O.M., Junjappa H., Ila H., J. Chem. Soc. Perkin Trans. 1 1997 356.
4.Jones G., in Comprehensive Heterocyclic Chemistry II, Katritzky A.R., Rees C.W., Scriven E.F.V., Eds., Oxford: Pergamon Press, 1996, vol. 5, p. 167.
5.Kim D.W., Choi H.Y., Lee K.-J., Chi D.Y., Org. Lett. 2001 3 445.
6.Paul S., Gupta M., Gupta R., Loupy A., Tetrahedron Lett. 2001 42 3827.
7.Su M., Liu Y., Ma H., Ma Q., et al., Chem. Commun. 2001 960.
8.Kerry M.A., Boyd G.W., Mackay S.P., Meth-Cohn O., Platt L., J. Chem. Soc. Perkin Trans. 1 1999 2315.
9.Ooi, T., Furuya M., Sakai D., Hokke Y., Maruoka K., Synlett 2001 541.
254 |
Устные доклады |
6-Метил-3,4-диоксо-1H-фуро[3,4-c]пиридин – синтетический аналог алкалоида cerpegin
Кайгородова Е.A.
Кубанский государственный технологический университет 350072, Краснодар, ул. Московская, 2
6-Метил-3,4-диоксо-1Н-фуро[3,4-c]пиридин 1 и продукты его превращения известны как потенциальные биологически активные вещества. Интерес к 1 обусловлен его сходством по строению с природным алкалоидом cerpegin 2 [1] и возможностью использования 1 как полупродукта в синтезе витамина В6 – пиридоксина 3
[2, 3].
|
|
|
2 |
|
|
|
|
|
OH |
|
||
|
1 |
|
O 3 |
|
O |
HO |
|
|||||
7 |
|
|
|
|
|
O |
|
|
O |
OH |
||
|
|
4 |
|
|
|
|
|
|
||||
6 |
|
|
|
|
|
|
|
|
|
|||
5 |
|
|
O |
N |
O |
|
N |
|
||||
|
|
N |
|
|
|
|
||||||
|
|
H |
|
|
|
|
H |
|
|
|
|
|
|
1 |
|
|
|
|
2 |
|
|
3 |
|
||
Фуропиридин 1 и его N-алкилпроизводные 4 получены гидролизом соответ- |
||||||||||||
ствующих пиридонов 5 [3, 4] (схема 1). |
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
Схема 1 |
|
|
|
O |
|
|
|
|
O |
|
|||
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
H2O |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
N O |
H2SO4 (50%) |
N O |
|
||||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
R |
|
||
|
|
|
|
|
5 R = H, Me, Bz |
|
|
|
1 R = H; |
|
||
|
|
|
|
|
|
|
|
|
|
4 R = Me, Bz |
|
|
Превращения соединения 1 могут быть разделены на два типа:
1)реакции, протекающие с сохранением лактонного цикла,
2)реакции, протекающие с раскрытием лактонного цикла.
Среди реакций первого типа исследованы реакции фуропиридина 1 с различными реагентами (схема 2):
–нитрование 1 нитрующей смесью дает 7-нитропроизводное 6 [3];
–кипячение 1 с PCl5 в растворе POCl3 приводит к образованию 4-хлорпири- дина 7 [2];
Генеральный спонсор и организатор – InterBioScreen Ltd. |
255 |
–бромирование 1 бромом в ледяной уксусной кислоте позволяет получить 7-бромпроизводное 8 [3].
Схема 2
|
|
|
O |
|
|
|
|
O2N |
|
O |
|
|
O |
N |
|
O |
O |
|
O |
H |
|
|
O |
|
6 |
|
|
||
N |
O |
|
O |
N |
Cl |
H |
|
|
|
7 |
|
|
|
Br |
|
O |
|
|
|
|
|
||
|
|
N |
|
O |
|
|
|
H |
|
|
|
|
|
8 |
|
|
|
При взаимодействии 1 с P4S10 образуется трудноразделимая смесь продуктов. Реакция сопровождается сильным осмолением. В то же время, замещение амидного кислорода на серу легко осуществить в две стадии (схема 3):
1)синтез 4-хлорпроизводного 7,
2)реакция 7 с тиомочевинной в бутаноле (или изопропаноле) с последующим разложением изотиурониевой соли 9 без ее выделения дает тион 10 [5].
Схема 3
S |
O |
|
O |
H2N |
O |
t |
O |
NH2 |
|
||
7 |
N S Cl− |
|
|
|
N |
S |
|
|
H2N + NH2 |
H |
|
|
|
|
|
|
9 |
10 |
|
Конденсация лактонов 1 и 10 с ароматическими и гетероароматическими альдегидами в кипящем бутаноле в присутствии вторичных аминов приводит к образованию илиденпроизводных 11 с хорошими выходами [5, 6] (схема 4).
256 |
Устные доклады |
