

12. Advances in the chemistry of amino and nitro compounds |
613 |
||
Ph |
|
Ph |
|
|
− |
|
|
|
R |
|
|
|
|
R |
|
|
|
|
|
NO2 − |
|
NO2 − |
|
(414) |
(415) |
|
|
3. Reactions of nitroalkenes
Thermolysis of allylic nitro compounds results in the formation of rearranged allyl alcohols; the cyclohexene 416, for instance, affords a 4:1 mixture of the cyclohexanol derivatives 417 and 418. It is proposed that the process involves a [2,3] sigmatropic shift of a nitro group (equation 137)451.
|
|
Me |
H |
Me |
H |
|
|
|
|
||
|
|
|
N |
O |
OH |
|
|
H |
O |
|
|
Me |
|
|
|
||
|
|
|
|
||
C |
+ O |
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
O− |
|
|
|
|
|
|
|
|
(417) |
|
|
H |
Me |
|
(137) |
|
|
H |
Me |
||
|
(416) |
N |
O |
|
|
|
O |
|
OH |
||
|
|
|
|
||
(418)
Substitution reactions of allylic nitro compounds often lead to rearranged products, as in palladium(0)-catalysed aminations and alkylations. Thus treatment of the nitro ester 419 with piperidine in the presence of tetrakis(triphenylphosphine)palladium yields a mixture of the unrearranged and rearranged amines 420 (R D piperidin-1-yl) and 421
CO2 Me |
CO2 Me |
Me NO2 |
Me R |
(419) |
(420) |
R |
CO2 Me |
+ |
|
|
Me |
(421)
614 |
G. V. Boyd |
(R D piperidin-1-yl), respectively. Under similar conditions, the same ester reacts with sodium benzenesulphinate to give a 95:5 mixture of the kinetic product 420 (R D O2SPh) and the thermodynamic product 421 (R D O2SPh)452.
The carbanion derived from dimethyl malonate reacts with the cyclic nitro compounds 422 of ring size 5, 6, 7, 8 and 12 to afford the corresponding esters 423. Acyclic allylic nitro compounds 424 (R D Me, CH2OAc or CO2Et) are attacked by bulky nucleophiles, such as dimethyl malonate anion, mainly at the terminal primary carbon atom to give rearranged products 425, whereas smaller nucleophiles, e.g. the anion derived from methyl cyanoacetate, react at the tertiary carbon atom to yield 426409a,453 455.
|
|
CO2 Me |
CH2 NO2 |
|
CH2 CH |
|
|
CO2 Me |
|
|
|
|
|
(422) |
|
(423) |
|
|
R |
MeO2 C |
|
R |
CN |
|
R |
CH |
|||
|
|
|
|||
|
|
HC |
|
||
|
NO2 |
|
|
CO2 Me |
|
Me |
MeO2 C |
Me |
|
||
|
Me |
|
|||
|
|
|
|
||
|
|
|
|
|
|
(424) |
|
|
(425) |
(426) |
|
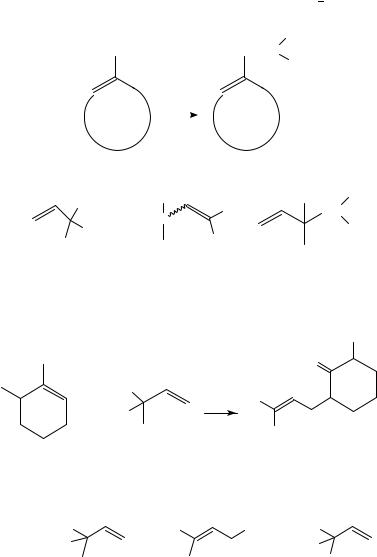
Another example of the formation of a rearranged product is the palladium(0)-catalysed reaction of the enolate ion of 2-methylcyclohexanone with 3-methyl-3-nitro-1-butene (equation 138)456.
|
|
Me |
OLi |
|
O |
Me |
Me |
(138) |
+ |
|
Me |
Me |
|
|
|
NO2 |
Me |
3-Methyl-3-nitro-1-nonene (427) and sodium phenylthiolate show different modes of behaviour under different conditions: in HMPA the product 428 of attack at the less hindered carbon atom is obtained, whereas in the presence of Pd(PPh3)4 the isomer 429 is produced457.
H13 C6 |
H13 C6 |
SPh H13 C6 |
Me |
|
Me |
NO2 |
Me |
SPh |
(427) |
(428) |
(429) |

12. Advances in the chemistry of amino and nitro compounds |
615 |
The allylic nitroalkenes 430 [R1 D R2 D Me; R1 D Me, R2 D CO2Et; R1R2 D CH2 4] react with lithium dialkylcuprates R32CuLi (R3 D Bu or Ph), obtained from organolithium compounds and copper(I) iodide, to yield the rearranged olefins 431458.
R1 |
R1 |
|
R2 |
+ R3 2 CuLi |
|
NO2 |
R2 |
R3 |
(430) |
|
(431) |
Under basic conditions, ˛-nitroalkenes function as synthetic equivalents of allylic nitro compounds; 3-nitro-3-hexene, for instance, reacts with piperidine in the presence of Pd(PPh3)4, to give 2-piperidinyl-3-hexene (equation 139)459.
|
NO2 |
|
NO2 |
Me |
Me |
Me |
Me |
|
|
|
HN |
|
|
|
(139) |
|
|
Me |
Me |
|
|
|
|
|
|
N |
|
The two-fold Michael addition of nitroethane to methyl propiolate in the presence of potassium fluoride and the phase-transfer catalyst tetrabutylammonium chloride leads to the diester 432. Treatment of nitroethane with methyl propiolate under these conditions, followed by methyl vinyl ketone, leads to the ‘mixed’ adduct 433460.
|
|
MeO2 C |
Me |
|
|
|
CO2 Me |
||
+ 2HC CCO2 Me |
|
|
||
|
|
|
NO2 |
|
MeCH2 NO2 |
+ HC CCO2 Me |
(432) |
||
O |
||||
|
||||
|
|
|
Me |
|
|
+ H2 C CHCOMe |
Me |
CO2 Me |
|
|
|
|||
NO2
(433)
Oximes RCHDNOH are produced in the reduction of nitroalkanes RCH2NO2 by carbon disulphide in the presence of triethylamine461 or wet potassium carbonate and

616 |
G. V. Boyd |
a phase-transfer agent462. Best yields are obtained from allylic nitro compounds and arylnitromethanes. When further carbon disulphide and aqueous sodium hydroxide are added, nitriles RCN result463. Conjugated nitroalkenes are reduced by sodium borohydride in methanol/THF to saturated nitro compounds, e.g. PhCHDCHNO2 ! PhCH2CH2NO2, while BH3 Ð THF, generated from sodium borohydride and boron trifluoride etherate in THF, affords the hydroxylamine PhCH2CH2NHOH464.
The ˇ, -epoxy nitro derivatives 435, prepared by oxidation of allylic nitro compounds, e.g. 434, with 3-chloroperbenzoic acid, undergo ring-opening on treatment with triethylamine in wet acetonitrile to yield the nitrovinyl alcohols 436, which arrange to the allylic nitro alcohols 437 on heating. In contrast, reaction of the epoxides with the soft nucleophiles piperidine or sodium benzenesulphinate in the presence of palladium tetrakis(triphenylphosphine) results in ring-scission and replacement of the nitro group to give compounds 438465.
|
|
NO2 |
|
NO2 |
|
NO2 |
NO2 |
HO |
|
|
HO |
|
|
||||
|
|
|
|
||
|
O |
−OH |
|
|
|
|
( )n |
|
( )n |
||
|
|
|
|||
|
|
(436) |
|
(437) |
|
( )n |
( )n |
− |
|
|
|
|
|
O2 SPh |
O2 SPh |
||
(434) |
(435) |
|
|
||
|
|
|
|
||
|
|
HO |
|
|
|
|
|
( |
)n |
||
|
|
(438) |
|
||
IV. REFERENCES
1.(a) S. Patai (Ed.), The Chemistry of the Amino Group, Wiley, London, 1968.
(b)H. Feuer (Ed.), The Chemistry of the Nitro and Nitroso Groups. Part 1, Wiley, New York, 1969.
(c)H. Feuer (Ed.), The Chemistry of the Nitro and Nitroso Groups. Part 2, Wiley, New York, 1970.
(d)S. Patai (Ed.), Supplement F: The Chemistry of Amino, Nitroso and Nitro Compounds and Their Derivatives, Wiley, Chichester, 1982.
2.N. M. Yoon, H. W. Lee, J. Choi and H. J. Lee, Bull. Korean Chem. Soc., 14, 281 (1993).
3.M. K. Park, D. G. Jang and B. H. Han, Bull. Korean Chem. Soc., 12, 709 (1991).
4.K. Kaneda, H. Kuwahara and T. Imanaka, J. Mol. Catal., 88, L267 (1994).
5.W. Baik, J. L. Han, K. C. Lee, N. H. Lee, B. H. Kim and J.-T. Hahn, Tetrahedron Lett., 35, 3965 (1994).
6.J. R. Hwu, F. F. Wong and M. J. Shiao, J. Org. Chem., 57, 5254 (1992).
7.I. Suzuki and Y. Hanazaki, Chem. Lett., 549 (1986).
8.Y. Kodera, S. Watanabe, Y. Imada and S.-I. Murahashi, Bull Chem. Soc. Jpn., 67, 2542 (1994).
9.M. A. Schwartz, J. Gu and X. Hu, Tetrahedron Lett., 33, 1687 (1992).
10.M. A. Schwartz and X. Hu, Tetrahedron Lett., 33, 1689 (1992).
11.A. Yoshimura, K. Asada and S. Oae, Bull. Chem. Soc. Jpn., 55, 3000 (1982).
12.M. F. Zipplies, M.-J. De Vos and T. C. Bruice, J. Org. Chem., 50, 3228 (1985).
13.J. P. Leeds and H. A. Kirst, Synth. Commun., 18, 777 (1988).
12. Advances in the chemistry of amino and nitro compounds |
617 |
14.S. Itsuno, Y. Sakurai, K. Shimizu and K. Ito, J. Chem. Soc., Perkin Trans. 1, 1548 (1989).
15.Y. Sakito, Y. Yoneyoshi and G. Suzukamo, Tetrahedron Lett., 29, 223 (1988).
16.D. E. Gibbs and D. Barnes, Tetrahedron Lett., 31, 5555 (1990).
17.A. S. B. Prasad, J. V. B. Kanth and M. Periasamy, Tetrahedron, 48, 4623 (1992).
18.G. B. Fisher, J. C. Fuller, J. Harrison, C. T. Goralski and B. Singaram, Tetrahedron Lett., 34, 1091 (1993).
19.M. Bonnat, A. Hercouet and M. LeCorre,´ Synth. Commun., 21, 1579 (1991).
20.S. Sengupta, D. P. Sahu and S. K. Chatterjee, Indian J. Chem., Sect. B, 33B, 285.
21.S. Akabori and Y. Takanohashi, Chem. Lett., 251 (1990).
22.S. Akabori, Y. Takanohashi, S. Aoki and S. Sato, J. Chem. Soc., Perkin Trans. 1, 3121 (1991).
23.A. Galan, J. De Mendoza, P. Prados, J. Rojo and A. M. Echavarren, J. Org. Chem., 56, 452 (1991).
24.E. F. V. Scriven and K. Turnbull, Chem. Rev., 88, 298 (1988).
25.A. A. Malik, S. B. Preston, T. G. Archibald, M. P. Cohen and K. Baum, Synthesis, 450 (1989).
26.S. N. Maiti, M. P. Singh and R. G. Micetich, Tetrahedron Lett., 27, 1423 (1986).
27.H. S. P. Rao and P. Siva, Synth. Commun., 24, 549 (1994).
28.S. N. Maiti, P. Spevak and A. V. N. Reddy, Synth. Commun., 18, 1201 (1988).
29.J. C. Sarma and R. P. Sharma, Chem. Ind. (London), 764 (1987).
30.N. M. Yoon, J. Choi and Y. S. Shon, Synth. Commun., 23, 3047 (1993).
31.A. Guy, A. Lemor, J. Doussot and M. Lemaire, Synthesis, 900 (1988).
32.A. Koziara, K. Osowska-Pacewicka, S. Zawadzki and A. Zwierzak, Synthesis, 202 (1985).
33.K. R. Gee and J. F. W. Keana, Synth. Commun., 23, 357 (1993).
34.S. Mori, T. Aoyama and T. Shioiri, Chem. Pharm. Bull., 34, 1524 (1986).
35.E. Fabiano, B. T. Golding and M. M. Sadeghi, Synthesis, 190 (1987).
36.D. H. R. Barton, J. P. Finet and J. Khamsi, Tetrahedron Lett., 28, 887 (1987).
37.D. H. R. Barton, N. Ozbalik and M. Ramesh, Tetrahedron Lett., 29, 857 (1988).
38.M. Deeba, M. E. Ford and T. A. Johnson, J. Chem. Soc., Chem. Commun., 562 (1987).
39.P. Dalla Croce, C. La Rosa and A. Ritieni, J. Chem. Res., Synop., 346 (1988).
40.W. ten Hoeve, C. G. Kruse, J. M. Luteyn, J. R. G. Thiecke and H. Wynberg, J. Org. Chem., 58, 5101 (1993).
41.Y.-S. Hon and L. Lu, Tetrahedron Lett., 34, 5309 (1993).
42.E. J. Corey and A. W. Gross, Tetrahedron Lett., 25, 491 (1984).
43.R. F. Smith, J. L. Marcucci and P. S. Tingue, Synth. Commun., 22, 381 (1992).
44.A. Koziara, K. Osowska-Pacewicka, S. Zawadzki and A. Zwierzak, Synthesis, 487 (1987).
45.F. Guennouni, F. Szoenyi and A. Cambon, Synth. Commun., 24, 2653 (1994).
46.U. Ragnarsson and L. Grehn, Acc. Chem. Res., 24, 285 (1991).
47.Y. Han and H. Hu, Synthesis, 122 (1990).
48.A. Zwierzak and S. Pilichowska, Synthesis, 922 (1982).
49.R. O. Hutchins, J. Wei and S. J. Rao, J. Org. Chem., 59, 4007 (1994).
50.A. Hosomi, S. Kohra, Y. Tominaga and M. Inaba, Chem. Pharm. Bull., 36, 2342 (1988).
51.L. Grehn and U. Ragnarsson, Synthesis, 275 (1987).
52.C. T. Clarke, J. D. Elliott and J. H. Jones, J. Chem. Soc., Perkin Trans. 1, 1088 (1978).
53.L. Grehn and U. Ragnarsson, Collect. Czech. Chem. Commun., 53, 2778 (1988).
54.R. D. Connell, T. Rein, B. Akermark and P. Helquist, J. Org. Chem., 53, 3845 (1988).
55.A. Koziara, J. Chem. Res., Synop., 296 (1989).
56.O. Mitsunobu and M. Yamada, Bull. Chem. Soc. Jpn., 40, 2380 (1967).
57.O. Mitsunobu and M. Eguchi, Bull. Chem. Soc. Jpn., 44, 3427 (1971).
58.M. L. Edwards, D. M. Stemerick and J. R. McCarthy, Tetrahedron Lett., 31, 3417 (1990).
59.D. M. Roundhill, Chem. Rev., 92, 1 (1992).
60.S. Ganguly and D. M. Roundhill, Polyhedron, 9, 2517 (1990).
61.K. T. Huh, S. C. Shim and C. H. Doh, Bull. Korean Chem. Soc., 11, 45 (1990).
62.J. A. Marsella, J. Org. Chem., 52, 467 (1987).
63.W. A. Nugent and R. L. Harlow, J. Am. Chem. Soc., 116, 6142 (1994).
64.M. Fujiwara, M. Imada, A. Baba and H. Matsuda, Tetrahedron Lett., 30, 739 (1989).
65.M. Chini, P. Crotti and F. Macchia, J. Org. Chem., 56, 5939 (1991).
66.R. K. Atkins, J. Frazier, L. L. Moore and L. O. Weigel, Tetrahedron Lett., 27, 2451 (1986).
67.J. Yamada, M. Yumoto and Y. Yamamoto, Tetrahedron Lett., 30, 4255 (1989).
68.M. Chini, P. Crotti. L. Favero, F. Macchia and M. Pineschi, Tetrahedron Lett., 35, 433 (1994).
618 |
G. V. Boyd |
69.D. Hurtaud, M. Baudy-Floc’h, A. Robert and P. LeGrel, J. Org. Chem., 59, 4701 (1994).
70.M. Canas, M. Poch, X. Verdaguer, A. Moyano, M. A. Pericas and A. Riera, Tetrahedron Lett., 32, 6931 (1991).
71.W. Chamchaang and A. R. Pinhas, J. Org. Chem., 55, 2531 (1990).
72.P. Crotti, L. Favero, F. Macchia and M. Pineschi, Tetrahedron Lett., 35, 7089 (1994).
73.P. Mangeney, A. Tejero, A. Alexakis, F. Grosjean and J. Normant, Synthesis, 255 (1988).
74.(a) C. A. Willoughby and S. L. Buchwald, J. Am. Chem. Soc., 114, 7562 (1992).
(b)C. A. Willoughby and S. L. Buchwald, J. Org. Chem., 58, 7627 (1993).
75.H. Okamoto and S. Kato, Bull. Chem. Soc. Jpn., 64, 3466 (1991).
76.A. R. Katritzky, M. Drewniak and J. M. Aurrocoechea, J. Chem. Soc., Perkin Trans. 1, 2359 (1987).
77.W. Dai, R. Srinivasan and J. A. Katzenellenbogen, J. Org. Chem., 54, 2204 (1989).
78.V. A. Soloshonok, A. G. Kirilenko, V. P. Kukhar and G. Resnati, Tetrahedron Lett., 35, 3119 (1994).
79.M. T. Reetz, R. Jaeger, R. Drewlies and M. Hubel,¨ Angew. Chem., Int. Ed. Engl., 30, 103 (1991).
80.D. H. R. Barton, J. C. Jaszberenyi and E. A. Theodorakis, J. Am. Chem. Soc., 114, 5904 (1992).
81.P. Andreoli, L. Billii, G. Cainelli, M. Panunzio, G. Martelli and G. Spunta, J. Org. Chem., 55, 4199 (1990).
82.E. J. Roskamp and S. F. Pedersen, J. Am. Chem. Soc., 109, 3152 (1987).
83.E. J. Enholm, D. C. Forbes and D. P. Holub, Synth. Commun., 20, 981 (1990).
84.R. G. Lovey and A. B. Cooper, Synlett, 167 (1994).
85.V. M. Vinogradov, I. L. Dalinger and S. A. Shevedlev, Mendeleev Commun., 111 (1993).
86.(a) I. Zaltsgendler, Y. Leblanc and M. A. Bernstein, Tetrahedron Lett., 34, 2441 (1993).
(b)H. Mitchell and Y. Leblanc, J. Org. Chem., 59, 682 (1994).
87.G. A. Olah, P. Ramaiah, Q. Wang and G. K. S. Prakash, J. Org. Chem., 58, 6900 (1993).
88.G. A. Olah and T. D. Ernst, J. Org. Chem., 54, 1203 (1989).
89.(a) H. Takeuchi, T. Adachi and H. Nishiguchi, J. Chem. Soc., Chem. Commun., 1524 (1991).
(b)H. Takeuchi, T. Adachi, H. Nishiguchi, K. Itou and K. Koyama, J. Chem. Soc., Perkin Trans. 1, 867 (1993).
90.H. Takeuchi, D. Higuchi and T. Adachi, J. Chem. Soc., Perkin Trans. 1, 1525 (1991).
91.H. Takeuchi, S. Hayakawa, T. Tanahashi, A. Kobayashi, T. Adachi and D. Higuchi, J. Chem. Soc., Perkin Trans. 2, 847 (1991).
92.J. P. Genet, S. Mallart, C. Greck and E. Piveteau, Tetrahedron Lett., 32, 2359 (1991).
93.A. Casarini, P. Dembech, D. Lazzari, E. Marini, G. Reginato, A. Ricci and G. Seconi, J. Org. Chem., 58, 5620 (1993).
94.T. Okawara, Y. Kanazawa, T. Yamasaki and M. Furukawa, Synthesis, 183 (1987).
95.G. Boche, C. Meier and W. Kleemiss, Tetrahedron Lett., 29, 1777 (1988).
96.G. Sosnovsky and K. Purgstaller, Z. Naturforsch., B: Chem. Sci., 44, 582 (1989).
97.V. V. Kuzmenko and A. F. Pozharskii, Zh. Org. Khim., 28, 1320 (1992); Chem. Abstr., 118, 169 046k (1993).
98.Y. Tamura, J. Minamikawa and M. Ikeda, Synthesis, 1 (1977).
99.M. J. Miller and G. M. Loudon, J. Org. Chem., 40, 126 (1975).
100.(a) H. C. Brown, W. R. Heydkamp, E. Breuer and W. S. Murphy, J. Am. Chem. Soc., 86, 3565 (1964).
(b)M. W. Rathke, N. Inoue, K. R. Warma and H. C. Bown, J. Am. Chem. Soc., 88, 2870 (1966).
101.Y. Tamura, J. Minamikawa, S. Fujii and M. Ikeda, Synthesis, 196 (1974).
102.P. Kovacic, M. K. Lowry and K. W. Field, Chem. Rev., 70. 639 (1970).
103.E. Erdik and M. Ay, Chem. Rev., 89, 1947 (1989).
104.G. Boche, N. Mayer, M. Bernheim and K. Wagner, Angew. Chem., Int. Ed. Engl., 17, 687 (1978).
105.S. Andreae and E. Schmitz, Synthesis, 327 (1991).
106.J. Vidal, L. Guy, S. Sterin and A. Collet, J. Org. Chem., 58, 4791 (1993).
107.V. F. Rudchenko, S. M. Ignatov and R. G. Kostyanovskii, J. Chem. Soc., Chem. Commun., 261 (1990).
108.(a) H. J. Bestmann and G. Wolfel,¨ Angew. Chem., Int. Ed. Engl., 23, 53 (1984).
(b)H. J. Bestmann, G. Wolfel¨ and K. Mederer, Synthesis, 848 (1987).
109.T. Morimoto, T. Takahashi and M. Sekiya, J. Chem. Soc., Chem. Commun., 794 (1984).
110.G. F. Grillot and R. E. Schaffrath, J. Org. Chem., 24, 1035 (1959).
111.J. J. Eisch, J. F. McNulty and X. Shi, J. Org. Chem., 59, 7 (1994).
12. Advances in the chemistry of amino and nitro compounds |
619 |
112.A. R. Katritzky, B. Pilarski and L. Urogdi, J. Chem. Soc., Perkin Trans. 1, 541 (1990).
113.A. R. Katritzky, S. Rachwal and B. Rachwal, J. Chem. Soc., Perkin Trans. 1, 791 (1987).
114.A. R. Katritzky, S. Rachwal and B. Rachwal, J. Chem. Soc., Perkin Trans. 1, 799 (1987).
115.(a) A. R. Katritzky, K. Yannakopoulou, W. Kuzmierkiewicz, J. M. Aurrocoechea, G. J. Palenik, A. E. Koziol, M. Szczesniak and R. Skarjune, J. Chem. Soc., Perkin Trans. 1, 2673 (1987).
(b)A. R. Katritzky, Q.-H. Long, P. Lue and A. Joswiak, Tetrahedron, 46, 8153 (1990).
(c)A. R. Katritzky and K. Yannakopoulou, Heterocycles, 28, 1121 (1989).
116.A. R. Katritzky, S. Rachwal and B. Rachwal, J. Chem. Soc., Perkin Trans. 1, 805 (1987).
117.A. R. Katritzky, K. Yannakopoulou, P. Lue, D. Rasala and L. Urogdi, J. Chem. Soc., Perkin Trans. 1, 225 (1989).
118.A. R. Katritzky, S. Rachwal and J. Wu, Can. J. Chem.,, 68, 456 (1990).
119.A. R. Katritzky, N. Shobana and P. A. Harris, Tetrahedron Lett., 32, 4247 (1991).
120.A. R. Katritzky, L. Urogdi and A. Mayence, Synthesis, 323 (1989).
121.A. R. Katritzky, J. K. Gallas and K. Yannakopoulou, Synthesis, 31 (1989).
122.A. R. Katritzky, X. Zhao and G. J. Hutchings, Synthesis, 703 (1991).
123.A. R. Katritzky and K. Yannakopoulou, Synthesis, 747 (1989).
124.J. Wang, H. Xu, X. Zhou, and S. Du, Youji Huaxue, 12, 198 (1992); Chem. Abstr., 116, 255 247s (1992).
125.J. Wang, S. Du, W. Bao and X. Zhou, Huaxue Shiji, 15, 19, 30 (1993); Chem. Abstr., 119, 72 290t (1993).
126.A. R. Katritzky, G. Noble, B. Pilarski and P. A. Harris, Chem. Ber., 123, 1443 (1990).
127.A. R. Katritzky, J. Jiang and P. A. Harris, Can. J. Chem., 69, 1153 (1991).
128.A. R. Katritzky and J.-J. Vanden Eynde, J. Chem. Soc., Perkin Trans. 1, 639 (1989).
129.A. R. Katritzky, K. Yannakopoulou and H. Lang, J. Chem. Soc., Perkin Trans. 2, 1867 (1994).
130.A. R. Katritzky, M. Latif and L. Urogdi, J. Chem. Soc., Perkin Trans. 1, 667 (1990).
131.A. R. Katritzky, L. Urogdi and A. Mayence, J. Org. Chem., 55, 2206 (1990).
132.A. R. Katritzky, Q.-H. Long and P. Lue, Tetrahedron Lett., 32, 3597 (1991).
133.A. R. Katritzky, J. Jiang and L. Urogdi, Tetrahedron Lett., 30, 3303 (1989).
134.A. R. Katritzky, J. Jiang and L. Urogdi, Synthesis, 565 (1990).
135.R. Saladino, C. Crestini and R. Nicoletti, Heterocycles, 38, 567 (1994).
136.A. R. Katritzky, A. Bieniek and B. E. Brycki, Chem. Scr., 29, 33 (1989).
137.C.-K. Chen, A. G. Hortmann and M. R. Marzabadi, J. Am. Chem. Soc., 110, 4829 (1988).
138.C. K. McGill and A. Rappa, Adv. Heterocycl. Chem., 44, 1 (1988).
139.H. Vorbruggen,¨ Adv. Heterocycl. Chem., 49, 117 (1990).
140.(a) H. C. Van der Plas, Janssen Chem. Acta, 3, 23 (1985).
(b)H. C. Van der Plas, Khim. Geterotsikl. Soedin., 1011 (1987); Chem. Abstr., 108, 150 331w (1988).
(c)M. Wozniak and H. C. Van der Plas, Acta Chem. Scand., 47, 95 (1993).
141.H. Tondys and H. C. Van der Plas, J. Heterocycl. Chem., 23, 621 (1986).
142.M. Wozniak, A. Baranski and B. Szpakiewicz, Justus Liebigs Ann. Chem., 875 (1991).
143.M. Wozniak and B. Szpakiewicz, Pol. J. Chem., 65, 1303 (1991).
144.M. Wozniak, K. Nowak and H. Poradowska, Pol. J. Chem., 65, 1077 (1991).
145.H. Sladowska, A. Van Veldhuizen and H. C. Van der Plas, J. Heterocycl. Chem., 23, 843 (1986).
146.T. Yamashita, K. Yamano, M. Yasuda and K. Shima, Chem. Lett., 627 (1993).
147.T. Yamashita, M. Yasuda, T. Isami, S. Nakano, K. Tanabe and K. Shima, Tetrahedron Lett., 34, 5131 (1993).
148.K. Yoshida, M. Hikasa, K. Ishii, H. Kadota and Y. Yamashita, J. Chem. Soc., Chem. Commun., 758 (1986).
149.M. Yasuda, T. Yamashita, K. Shima and C. Pac, J. Org. Chem., 52, 753 (1987).
150.M. Yasuda, K. Shiomori, S. Hamasuna, K. Shima and T. Yamashita, J. Chem. Soc., Perkin Trans. 2, 305 (1992).
151.S. Bhattacharyya, A. Chatterjee and S. K. Duttachowdhury, J. Chem. Soc., Perkin Trans. 1, 1 (1994).
152.P. Lue, J. Li, G. Li and X. Zhou, Youji Huaxue, 6, 447 (1986); Chem. Abstr., 107, 133 591t (1987).
153.R. Wang and Y. Guan, Gaodeng Xuexiao Huaxue Xuebao, 11, 894 (1990); Chem. Abstr., 114, 101 879w (1991).
154.R. F. Borch, M. D. Bernstein and H. D. Durst, J. Am. Chem. Soc., 93, 2897 (1971).
620 |
G. V. Boyd |
155.N. M. Yoon, E. G. Kim, H. S. Son and J. Choi, Synth. Commun., 23, 1595 (1993).
156.A. F. Abdel-Magid, C. A. Maryanoff and K. G. Carson, Tetrahedron Lett., 31, 5595 (1990).
157.C. L. Barney, E. W. Huber and J. R. McCarthy, Tetrahedron Lett., 31, 5547 (1990).
158.A. E. Moormann, Synth. Commun., 23, 789 (1993).
159.N. Kambe, T. Inagaki, N. Miyoshi, A. Ogawa and N. Sonoda, Chem. Lett., 1275 (1987).
160.I. V. Micovi´c,´ M. D. Ivanovic,´ D. M. Piatak and V. D. Bojic,´ Synthesis, 1043 (1991).
161.S. Bhattacharyya, Tetrahedron Lett., 35, 2401 (1994).
162.A. G. Giumanini, G. Chiavari, M. Musiani and P. Rossi, Synthesis, 743 (1980).
163.G. Verardo, A. G. Giumanini, P. Strazzolini and M. Poiana, Synthesis, 121 (1993).
164.A. Guy and J. F. Barbetti, Synth. Commun., 22, 853 (1992).
165.R. Carlson, T. Lejon, T. Lundstedt and E. LeClouerec, Acta Chem. Scand., 47, 1046 (1993).
166.R. Chauvin, Synth. Commun., 21, 1425 (1991).
167.S. K. Sharma, M. F. Songster, T. L. Colpitts, P. Hegyes, G. Barany and F. J. Castellino, J. Org. Chem., 58, 4993 (1993).
168.C. Boga, F. Manescalchi and D. Savoia, Tetrahedron, 50, 4709 (1994).
169.C. Betschart and D. Seebach, Helv. Chim. Acta, 70, 2215 (1987).
170.G. W. Kabalka, D. A. Henderson and R. S. Varma, Organometallics, 6, 1369 (1987).
171.G. W. Kabalka, N. M. Goudgaon and Y. Liang, Synth. Commun., 18, 1363 (1988).
172.H. C. Brown, W. R. Heydkamp, E. Breuer and W. S. Murphy, J. Am. Chem. Soc., 86, 3565 (1964).
173.G. W. Kabalka, K. A. R. Sastry, G. W. McCollum and H. Yoshioka, J. Org. Chem., 46, 4296 (1981).
174.M. Nakagawa, T. Kawate, T. Kakikawa, H. Yamada, T. Matsui and T. Hino, Tetrahedron, 49, 1739 (1993).
175.S. Itsuno, C. Hachisuka and K. Ito, J. Chem. Soc., Perkin Trans. 1, 1767 (1991).
176.H. C. Brown, M. M. Midland, A. B. Levy, A. Suzuki, S. Sono and M. Itoh, Tetrahedron, 43, 4079 (1987).
177.M. Vaultier, B. Carboni and P. Martinez-Fresneda, Synth. Commun., 22, 665 (1992).
178.J. P. Genet, J. Hajicek, L. Bischoff and C. Greck, Tetrahedron Lett., 33, 2677 (1992).
179.(a) G. W. Kabalka and Z. Wang, Organometallics, 8, 1093 (1989).
(b) G. W. Kabalka and Z. Wang, Synth. Commun., 20, 231 (1990).
180.R. Chaabouni, A. Laurent and B. Marquet, Tetrahedron, 36, 877 (1980).
181.N. A. Petasis and I. Akritopoulou, Tetrahedron Lett., 34, 583 (1993).
182.M.-J. Wu, D.-S. Yan, H.-W. Tsai and S.-H. Chen, Tetrahedron Lett., 35, 5003 (1994).
183.R. B. Cheikh, R. Chaabouni, A. Laurent, P. Mison and A. Nafti, Synthesis, 685 (1983).
184.G. S. Silverman, S. Strickland and K. M. Nicholas, Organometallics, 5, 2117 (1986).
185.Review: B. M. Trost, Tetrahedron, 33, 2615 (1977).
186.B. M. Trost and E. Keinan, J. Org. Chem., 44, 3451 (1979).
187.R. A. T. M. van Benthem, J. J. Michels, H. Hiemstra and W. N. Speckamp, Synlett, 368 (1994).
188.R. D. Connell, T. Rein, B. Aakermark and P. Helquist, J. Org. Chem., 53, 3845 (1988).
189.J. Barluenga, J. Perez-Prieto and G. Asensio, Tetrahedron, 46, 2453 (1990).
190.J. B. Baruah and A. G. Samuelson, Tetrahedron, 47, 9449 (1991).
191.N. DeKimpe, E. Stanoeva, R. Verhe´ and N. Schamp, Synthesis, 587 (1988).
192.K. J. M. Beresford, G. P. Howe and G. Procter, Tetrahedron Lett., 33, 3355 (1992).
193.P. J. Bhuyan, D. Prajapati and J. S. Sandhu, Tetrahedron Lett., 34, 7975 (1993).
194.M. Giammaruco, M. Taddai and P. Ulivi, Tetrahedron Lett., 34, 3635 (1993).
195.P. A. Grieco and A. Bahsas, J. Org. Chem., 52, 1378 (1987).
196.W. L. Neumann, M. M. Rogic and T. J. Dunn, Tetrahedron Lett., 32, 5865 (1991).
197.K. Takai, H. Odaka, Y. Kataoka and K. Utimoto, Tetrahedron Lett., 35, 1893 (1994).
198.M. Johannsen and K. A. Jørgensen, J. Org. Chem., 59, 214 (1994).
199.A. Srivastava, Y. Ma, R. Pankayatselvan, W. Dinges and K. M. Nicholas, J. Chem. Soc., Chem. Commun., 853 (1992).
200.K. B. Sharpless, T. Hori, L. K. Truesdale and C. O. Dietrich, J. Am. Chem. Soc., 98, 269 (1976).
201.K. B. Sharpless and T. Hori, J. Org. Chem., 41, 176 (1976).
202.J. P. Mahy, G. Bedi, P. Battioni and D. Mansuy, Tetrahedron Lett., 29, 1927 (1988).
203.F. Palacios, D. Aparicio and J. Garcia, Synlett, 260 (1994).
204.R. J. P. Corriou, G. Bolin and J. J. E. Moreau, Bull. Soc. Chim. Fr., 130, 273 (1993).
205.T. Murai, M. Yamamoto and S. Kato, J. Chem. Soc., Chem. Commun., 789 (1990).
12. Advances in the chemistry of amino and nitro compounds |
621 |
206.Y. Leblanc, R. Zamboni and M. A. Bernstein, J. Org. Chem., 56, 1971 (1991).
207.P. Kocovsky, Synlett, 677 (1990).
208.J. F. Dellaria, Jr. and K. J. Sallin, Tetrahedron Lett., 31, 2661 (1990).
209. R. G. Shea, J. N. Fitzner, J. E. Frankhauser, A. Spaltenstein, P. A. Carpino, R. M. Peevey,
D.V. Pratt, B. J. Tenge and P. B. Hopkins, J. Org. Chem., 51, 5243 (1986).
210.A. I. Meyers, J. P. Lawson and D. R. Carver, J. Org. Chem., 46, 3119 (1981).
211.A. M. Caporusso, R. Geri, C. Polizzi and L. Lardicci, Tetrahedron Lett. 32, 7471 (1991).
212.Y. Imada, M. Yuasa, I. Nakamura and S.-I. Murahashi, J. Org. Chem., 59, 2282 (1994).
213.J. Barluenga, P. J. Campos and G. Canal, Synthesis, 33 (1989).
214.L. Leboutet, G. Courtois and L. Miginiac, J. Organomet. Chem., 420, 155 (1991).
215.V. N. Komissarov, L.Yu. Ukhin, Zh. I. Orlova and O. A. Tokarskaya, Zh. Org. Khim., 23, 1325 (1987); Chem. Abstr. 108, 204 183v (1988).
216.J. R. Hauske, P. Dorff, S. Julin, G. Martinelli and J. Bussolari, Tetrahedron Lett., 33, 3715 (1992).
217.D. Damour, J. Pornet, B. Randrianoelina and L. Miginiac, J. Organomet. Chem., 396, 289 (1990).
218.P. Casara, K. Jund and P. Bey, Tetrahedron Lett., 25, 1891 (1984).
219.J. R. McCarthy, C. L. Barney, D. P. Matthews and T. M. Bargar, Tetrahedron Lett., 28, 2207 (1987).
220.N. E. Lee and S. L. Buchwald, J. Am. Chem. Soc., 116, 5985 (1994).
221.K. Van Sant and M. S. South, Tetrahedron Lett., 28, 6019 (1987).
222.F. G. West, K. W. Glaeske and B. N. Naidu, Synthesis, 997 (1993).
223.Y. Uozumi, M. Mori and M. Shibasaki, J. Chem. Soc., Chem. Commun., 81 (1991).
224.S. Tollari, M. Cuscela and F. Porta, J. Chem. Soc., Chem. Commun., 1510 (1993).
225.H. R. Sonawane, A. V. Pol, P. P. Moghe, S. S. Biswas and A. Sudalai, J. Chem. Soc., Chem. Commun., 1215 (1994).
226.M. Hadayatullah and A. Roger, Bull. Soc. Chim. Belg., 102, 59 (1993).
227.(a) C. Nallaiah and J. A. Strickson, Tetrahedron, 42, 4083 (1986).
(b)C. Nallaiah and J. A. Strickson, Tetrahedron, 42, 4089 (1986).
228.(a) R. W. Murray, R. Jeyaraman and L. Mohan, Tetrahedron Lett., 27, 2335 (1986).
(b)R. W. Murray, S. N. Rajadhyaksha and L. Mohan, J. Org. Chem., 54, 5783 (1989).
229.D. L. Zabrowski, A. E. Moormann and K. R. Beckwith, Jr., Tetrahedron Lett., 29, 4501 (1988).
230.M. Kol and S. Rozen, J. Chem. Soc., Chem. Commun., 567 (1991).
231.S. Rozen and M. Kol, J. Org. Chem., 57, 7342 (1992).
232.S. Murahashi, H. Mitsui, T. Shiota, T. Tsuda and S. Watanabe, J. Org. Chem., 55, 1736 (1990).
233.(a) R. W. Murray and M. Singh, Synth. Commun., 19, 3509 (1989).
(b)R. W. Murray and M. Singh, J. Org. Chem., 55, 2954 (1990).
234.S. M. Neset, T. Benneche and K. Undheim, Acta. Chem. Scand., 47, 1141 (1993).
235.J. K. Crandall and T. Reix, J. Org. Chem., 57, 6759 (1992).
236.S. Murahashi and T. Shiota, Tetrahedron Lett., 28, 2383 (1987).
237.W. W. Zajac, Jr., M. G. Darcy, A. P. Subong and J. H. Buzby, Tetrahedron Lett., 30, 6495 (1989).
238.W. W. Zajac, Jr., T. R. Walters and M. G. Darcy, J. Org. Chem., 53, 5856 (1988).
239.L. Grierson and M. J. Perkins, Tetrahedron Lett., 34, 7463 (1993).
240.J. S. Reddy and P. A. Jacobs, J. Chem. Soc., Perkin Trans. 1, 2665 (1993).
241.P. Capdevielle and M. Maumy, Tetrahedron Lett., 31, 3891 (1990).
242.G. A. Lee and H. H. Freedman, Isr. J. Chem., 26, 229 (1985).
243.Y. Hu and H. Hu, Synth. Commun., 22, 1491 (1992).
244.H. G. Chen and P. Knochel, Tetrahedron Lett., 29, 6701 (1988).
245.P. Muller¨ and D. M. Gilabert, Tetrahedron, 44, 7171 (1988).
246.S.-I. Murahashi, T. Naota and H. Taki, J. Chem. Soc., Chem. Commun., 613 (1985).
247.S. Yamazaki, Chem. Lett., 823 (1992).
248.J. Yamaguchi and T. Takeda, Chem. Lett., 1933 (1992).
249.A. Goti and M. Romani, Tetrahedron Lett., 35, 6567 (1994).
250.R. V. Hoffman and A. Kumar, J. Org. Chem., 49, 4011 (1984).
251.T. Kametani, K. Takahashi, T. Ohsawa and M. Ihara, Synthesis, 245 (1977).
252.(a) P. Capdevielle, A. Lavigne and M. Maumy, Synthesis, 453 (1989).
(b)P. Capdevielle, A. Lavigne, D. Sparfel, J. Baranne-Lafont, K. C. Nguyen and M. Maumy,
Tetrahedron Lett., 31, 3305 (1990).
622 |
G. V. Boyd |
253.B. Jursiˇc,´ J. Chem. Res., Synop., 168 (1988).
254.S. Yamazaki and Y. Yamazaki, Bull. Chem. Soc. Jpn.,, 63, 301 (1990).
255.J. B. Lee, C. Parkin, M. J. Shaw, N. A. Hampson and K. I. MacDonald, Tetrahedron, 29, 751 (1973).
256.W. Gottardi, Monatsh. Chem., 104, 1690 (1973).
257.J. Santamaria, R. Ouchabane and J. Rigaudy, Tetrahedron Lett., 30, 3977 (1989).
258.J. Santamaria, M. T. Kaddachi and J. Rigaudy, Tetrahedron Lett., 31, 4735 (1990).
259.T. H. Chuang, C. C. Yang, C. J. Chang and J. M. Fang, Synlett, 733 (1990).
260.J. H. Cooley and E. J. Evain, Synthesis, 1 (1989).
261.J. D. Hobson and J. G. McCluskey, J. Chem. Soc. (C), 2015 (1967).
262.T. A. Montzka, J. D. Matiskella and R. A. Partyka, Tetrahedron Lett., 1325 (1974).
263.R. A. Olofson, R. C. Schnur, L. Bunes and J. P. Pepe, Tetrahedron Lett., 1567 (1977).
264.R. A. Olofson, J. T. Martz, J.-P. Senet, M. Piteau and T. Malfroot, J. Org. Chem., 49, 2081 (1984).
265.S. Murahashi, T. Naota and K. Yonemura, J. Am. Chem. Soc., 110, 8256 (1988).
266.J. V. B. Kanth, C. K. Reddy and M. Periasamy, Synth. Commun., 24, 313 (1994).
267.S. Murahashi, T. Naota, N. Miyaguchi and T. Nakato, Tetrahedron Lett., 33, 6991 (1992).
268.S. Murata, K. Suzuki, A. Tamatani, M. Miura and M. Nomura, J. Chem. Soc., Perkin Trans. 1, 1387 (1992).
269.P. Nussbaum, K. Baumann, T. Dechat and M. Harasek, Tetrahedron, 47, 4591 (1991).
270.(a) G. Verardo, A. G. Giumanini and P. Strazzolini, Tetrahedron, 46, 4303 (1990).
(b)G. Verardo, A. G. Giumanini and P. Strazzolini, Tetrahedron, 47, 7845 (1991).
271.M. V. Vouk, Zh. Org. Khim., 23, 2023 (1987); Chem. Abstr., 109, 37 501j (1988).
272.R. Carlson, U. Larsson and L. Hansson, Acta Chem. Scand., 46, 1211 (1992).
273.R. M. Moriarty, T. E. Hopkins, I. Prakash, B. K. Vaid and R. K. Vaid, Synth. Commun., 20, 2353 (1990).
274.A. De Nicola, J. Einhorn and J.-L. Luche, J. Chem. Res., Synop., 278 (1991).
275.E. Juaristi, P. Murer and D. Seebach, Synthesis, 1243 (1993).
276.N. X. Hu, Y. Aso, T. Otsubo and F. Ogura, Tetrahedron Lett., 29, 4949 (1988).
277.C. D. Xu, S. M. Lu and X. Huang, Chin. Chem. Lett., 4, 869 (1993).
278.D. H. R. Barton, J. P. Finet and J. Khamsi, Tetrahedron Lett., 27, 3615 (1986).
279.D. H. R. Barton, D. M. X. Donnelly, J. P. Finet and P. J. Guiry, Tetrahedron Lett., 30, 1377 (1989).
280.S. Gauthier and J. M. J. Frechet, Synthesis, 383 (1987).
281.J. V. B. Kanth and M. Periasamy, J. Org. Chem., 58, 3156 (1993).
282.A. S. Guram and S. L. Buchwald, J. Am. Chem. Soc., 116, 7901 (1994).
283.R. F. Pellon, R. Carrasco and L. Roder, Synth. Commun., 23, 1447 (1993).
284.A. A. Galan, T. V. Lee and C. B. Chapleo, Tetrahedron Lett., 27, 4995 (1986).
285.(a) K. Mai and G. Patil, Synth. Commun., 15, 157 (1985).
(b)K. Mai and G. Patil, Org. Prep. Proced. Int., 17, 183 (1985).
286.J.-P. Leblanc and H. W. Gibson, Tetrahedron Lett., 33, 6295 (1992).
287.M. J. Genin, C. Biles and D. L. Romero, Tetrahedron Lett., 34, 4301 (1993).
288.M. Schorr and W. Schmitt, Phosphorus, Sulfur Silicon Relat. Elem., 68, 25 (1992).
289.K. N. Radhamani, A. J. Elias and D. K. Padma, Phosphorus, Sulfur Silicon Relat. Elem., 66, 297 (1992).
290.H. Heany, in Comprehensive Organic Synthesis (Ed. B. M. Trost), Vol. 2, Pergamon, Oxford, 1991, p. 953.
291.M. Lubben and B. L. Feringa, J. Org. Chem., 59, 2227 (1994).
292.S. Kohra, S. Tutuya, M. Kimura, K. Ogata and Y. Tominaga, Chem. Pharm. Bull., 41, 1293 (1993).
293.T. Satoh, Y. Kaneko, K. Sakata and K. Yamakawa, Bull. Chem. Soc. Jpn., 59, 457 (1986).
294.S. Husinec, I. Juranic, A. Llobera and A. E. A. Porter, Synthesis, 721 (1988).
295.D. Enders and M. Finkam, Synlett, 401 (1993).
296.N. DeKimpe, L. D’Hondt and L. Moens, Tetrahedron, 48, 3183 (1992).
297.K. Sato, M. Ohashi, E. Aoki and Y. Murai, J. Org. Chem., 42, 3713 (1977).
298.M. B. Gase, A. Lattes and J. J. Perie, Tetrahedron, 39, 703 (1983).
299.J.-J. Brunet, D. Neibecker and F. Niedercorn, J. Mol. Catal., 49, 235 (1989).
300.G. P. Pez and J. E. Galle, Pure Appl. Chem., 57, 1917 (1985).
