

12. Advances in the chemistry of amino and nitro compounds |
553 |
which afford primary amines R1NH2 on hydrolysis or reduction24. Primary amines are produced from Grignard reagents and arenediazonium tetrafluoroborates, followed by reduction of the resulting azo compounds (equation 38)103.
+ |
|
SnCl2/HCl |
|
RMgX C Ar N2 BF4 |
! RNDNAr ! RNH2 |
38 |
Electrophilic amination reactions by means of oxaziridines have been reviewed105. Cyclohexylidenehydrazines 86 are formed from secondary amines such as diethylamine, dibutylamine and morpholine and the spirooxaziridine 85105.
R2 NH + |
NH |
|
|
|
|
|
|
|
|
|
|
|
N NR2 |
||
|
− H2 |
O |
|
||||
|
|
|
|
|
|||
|
O |
|
|
|
|
|
|
|
(85) |
|
|
|
(86) |
|
|
2-Acyloxaziridines are unrivalled as acylamino |
transfer |
reagents, see e.g. |
|||||
equation 39106. |
|
|
|
|
|
|
|
O |
OBut |
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
|
N |
|
|
|
(39) |
||
|
+ HNR1R2 |
R1 R2 N |
|||||
|
|
NHCO2 But |
|||||
|
O |
|
|
|
|||
|
|
|
|
|
|
||
|
− NC |
|
CHO |
|
|
|
|
NC
N,N-Dimethoxyamine is a source of the N-methoxynitrenium ion, which is generated by the action of boron trifluoride etherate. In the presence of dimethylsulphide the salt 87 is obtained; triphenylphosphine yields the analogue 88107.
|
|
|
|
|
|
+ |
|
OMe |
|
|
|
Me2 |
S |
Me2 SNHOMe |
− |
+ |
|
|
BF4 |
||||
|
|
|
|
|
|||
HN |
HN |
|
OMe |
|
(87) |
|
|
|
|
|
|||||
OMe |
|
|
BF4 |
− |
P |
+ |
|
|
|
|
|
Ph3 |
Ph3 PNHOMe |
|
|
|
|
|
|
|
|
− |
|
|
|
|
|
|
|
BF4 |
|
|
|
|
|
|
|
(88) |
|
+
8. Via >N C < synthons
The disilylamines 90, prepared from the chloro ethers 89 (R1 D Me or C6H13)+and
sodium bis(trisilylmethyl)amide function as synthetic equivalents of the (Me3Si)2NCH2 cation. They react with Grignard reagents R2MgBr (R2 D Me, i-Pr, cyclohexyl, Ph, PhCH2, CH2DCHCH2 or C3H7C C) to give the silylated amines 91, which are hydrolysed to the corresponding amine hydrochlorides 92 by dilute hydrochloric acid108,109.

554 |
|
G. V. Boyd |
|
|
|
+ − |
|
2 |
|
|
+ Na N(SiMe3 )2 |
|
+R MgBr |
|
R1OCH2 Cl |
|
R1OCH2 N(SiMe3 )2 |
|
R2 CH2 N(SiMe3 )2 |
|
|
|||
|
|
|
−R1OMgBr |
|
(89) |
|
(90) |
(91) |
|
R2 CH2 NH2 . HCl
(92)
Similarly, aminomethyl sulphides (from amines, formaldehyde and mercaptans) react with organolithium compounds to afford amines (equation 40)110,111.
|
|
|
|
|
|
|
CR4Li |
|
R1SH |
C |
CH O |
C |
HNR2R3 |
! |
R1SCH NR2R3 |
! |
R4CH NR2R3 40 |
|
|
2 |
|
|
|
2 |
1 |
2 |
|
|
|
|
|
|
|
+ |
+ |
The synthetic utility of benzotriazole derivatives as >N C< $ >NDC< synthons has been explored by Katritzky and his coworkers. The Mannich reaction of benzotriazole with primary aliphatic amines RNH2 and formaldehyde in an aqueous medium results in the formation of one or more of three types of product: BtCH2NHR, (BtCH2)2NR and (BtCH2NR)2CH2 [Bt D benzotriazol-1-yl (93)]112. Benzotriazole and aliphatic or aromatic aldehydes yield compounds 94113. The parent compound 1- hydroxymethylbenzotriazole reacts with aromatic amines to give secondary amines 95, primary aliphatic amines give the tertiary amines 96 and aqueous ammonium acetate at room temperature yields 97. The 1-benzotriazoles 98 are obtained from benzotriazole, aldehydes and aromatic amines114.
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
Bt |
|
CHROH |
Bt |
|
CH2 NHAr |
||||
|
|
||||||||||||
|
|
N |
|
|
(94) |
|
|
(95) |
|||||
|
|
|
|
|
|
|
|||||||
(93) |
|
|
|
|
|
|
|
|
|
|
|
||
Bt |
|
CH2 NR1 CH2 |
|
Bt |
|
(Bt |
|
CH2 )3 N |
|
Bt |
|
CHRNHAr |
|
|
|
|
|
|
|
||||||||
|
|
|
|||||||||||
(96) |
|
|
(97) |
(98) |
|||||||||
The products from benzotriazole, aldehydes and primary or secondary amines exist in the melt or in solution as equilibrium mixtures of 1- and 2-benzotriazolyl compounds, 99 and 100, whereas the solids are 1-benzotriazoles 99115. The equilibrium involves the resonance-stabilized aminomethyl cation and the delocalized benzotriazolide anion; it accounts for the ease with which the bond attached to the benzotriazole moiety is cleaved.
Heating the equilibrium mixture obtained from benzotriazole, an aldehyde RCH2CHO (R D Me, Et, Pr or Bu) and morpholine with sodium hydride in THF results in the

12. Advances in the chemistry of amino and nitro compounds |
555 |
||||||||||
N |
|
|
|
N |
|
|
|
|
N |
|
|
N |
|
|
|
− N |
|
|
|
|
N |
CHR1 NR2 R3 |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
||||||
N |
+ |
N |
|
|
|
|
N |
|
|||
CHR1NR2 R3 |
|
|
|
|
|
(100) |
|
||||
|
|
|
|
|
|
|
|
|
|||
(99) |
+ |
|
|
|
|
|
|
|
+ |
|
|
CHR1 |
|
NR2 R3 |
|
|
|
CHR1 |
|
NR2 R3 |
|
||
|
|
|
|
|
|
||||||
|
|
|
|
|
|||||||
|
|
|
|
|
|
||||||
formation of an enamine with the elimination of sodium benzotriazolide and hydrogen (equation 41)115c.
CH2 R |
|
|
R |
H |
|
Bt CH N |
O |
+ NaH |
|
+ BtNa + H2 |
|
|
|
|
|||
|
|
|
H |
N |
(41) |
|
|
|
|
||
|
|
|
|
|
O |
The benzotriazole derivatives 101 (R1 D alkyl) formed from aliphatic aldehydes, benzotriazole and primary aromatic amines are reduced by lithium aluminium hydride or sodium borohydride to secondary amines 102, while Grignard reagents yield compounds of type 103116. The reaction has been used for the side-chain alkylation of 2-aminopyridine (equation 42) (direct alkylation occurs predominantly at the ring nitrogen atom).
|
|
|
R1CH2 NHAr |
1 |
(102) |
||
Bt |
|
CHR NHAr |
R2 MgX |
|
|||
|
|
|
|
|
|
|
R1R2 CHNHAr |
(101)(103)
|
Me |
|
Bt CHMeNH |
+ MeMgI |
|
CHNH |
(42) |
|
N |
Me |
N |
Numerous tertiary amines have been obtained from 104 (R1
R3 D alkyl) and sodium hydride or Grignard reagents (equation 43)116.
Bt CHR1NR2R3 ! CHR1R4NR2R3
(104)
The tertiary amines 96 react with organometallic compounds to afford disubstituted products 105117.
Bt CH2NR1CH2 Bt C 2R2M ! R2CH2NR1CH2R2
(96) (105)

556 |
G. V. Boyd |
Treatment of secondary amines 106 with hydroxymethylbenzotriazole yields 107, which forms tertiary amines 108 (Ar D Ph or 4-MeC6H4; R1, R2 D i-Pr, PhCH2 etc)118.
|
|
|
|
Ar |
|
Ar |
|
|
|
CH2 OH + ArNHCH2 R1 |
|
|
R2 |
MgX |
|
Bt |
|
|
Bt CH2 N |
|
|
R2 CH2 N |
|
|
|
|
|
||||
|
|
|
|
CH2 R1 |
|
CH2 R1 |
|
(106) |
(107) |
|
(108) |
||||
The benzotriazole 109 reacts with alkyl or allyl halides in the presence of bismuth(III) chloride and metallic aluminium to give the homoalkylated amines 110 in high yields119.
Bt CH2NMePh C RX ! RCH2NMePh
(109) (110)
The benzotriazole derivatives 111, obtained from benzotriazole, ethyl glyoxylate and secondary amines (diethylamine, pyrrolidine, piperidine or morpholine), furnish the amino esters 112 by the action of organozinc reagents R2ZnX (R2 D Me, Bu, PhCH2 or Ph)120.
Bt |
|
CHNR12 |
|
R2 CHNR12 |
||
|
|
|||||
|
|
|
|
|
|
|
CO2 Et |
CO2 Et |
|||||
(111) |
(112) |
|||||
Tertiary propargylamines 114 (R1 D H, Pr, i-Pr, C7H15 or Ph; NR22 D NMe2, NEt2, pyrrolidin-1-yl, piperidin-1-yl etc; R3 D C6H13, C8H17 or Ph) are formed from the benzotriazoles 113 and lithium acetylides121.
Bt CHR1NR22 |
C R3C CLi ! R3C CCHR1NR22 |
(113) |
(114) |
Hindered aliphatic aldehydes R1CHO (R1 D i-Pr or t-Bu) react with benzotriazole and anhydrous methanolic ammonia to yield the secondary amines 115, which are transformed into the phenylated amines 116 by the action of phenyllithium. Benzotriazole, aromatic aldehydes and ammonia give the imines 117, which react with lithium aluminium hydride to form dibenzylamines 118122.
PhLi
Bt CHR1NCHR1 Bt ! PhCHR1NCHR1Ph
H |
H |
(115) |
(116) |
|
LiAlH4 |
Bt CHArNDCHAr ! ArCH2NHCH2Ar
(117) (118)
The ‘Reformatsky reaction’ of the benzotriazoles 119 (R1 D H, Pr, i-Pr or Ph; NR22 D
pyrrolidin-1-yl, piperidin-1-yl or morpholin-4-yl) with the bromo esters 120 (R3, R4 D H or Me) in the presence of zinc affords the ˇ-amino esters 121123.

12. Advances in the chemistry of amino and nitro compounds |
557 |
||
Bt CHR1NR22 |
Zn |
|
|
C EtO2CCR3R4Br ! |
EtO2CCR3R4CHR1NR22 |
|
|
(119) |
(120) |
(121) |
|
Compounds 122, prepared from benzotriazole, p-dimethylaminobenzaldehyde and primary aromatic amines, are decomposed to the secondary amines 123 by sodium tetrafluoroborate124.
Bt CHNHAr CH2 NHAr
|
|
|
|
|
NMe2 |
|
|
|
|
NMe2 |
|
|
|
|
|
|
|
|
|
(122) |
|
|
|
(123) |
|
|
|
|
|
|
A variety of benzylamines 124 (R1 |
D |
Ph, Ar, 2-naphthyl, or 3- or 4-pyridyl etc; |
|||||||||||
NR |
2 |
|
|
|
|
|
|
|
|
|
|
|
||
|
2 D |
piperidin-1-yl or morpholin-4-yl) was obtained from benzotriazole, an aldehyde |
||||||||||||
1 |
|
|
2 |
|
|
|
|
|
|
|
|
|
||
R |
CHO and an amine HNR 2 and subsequent reduction of the products with sodium |
|||||||||||||
borohydride (equation 44)125. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Bt CHR1NR22 |
! R1CH2NR22 |
|
44 |
||||||
|
|
|
|
|
|
|
|
|
|
(124) |
|
|
|
|
N |
-Substitution of |
|
|
1NH |
(R1 |
D |
t-Bu, C H |
17 |
, cyclopentyl, |
|||||
|
primary aliphatic2 amines R1 |
(R |
22 |
|
8 |
|
||||||||
cyclohexyl, PhMeCH etc.) to yield R CH2NHR |
|
D Et, Ph or PhCH2) is accomplished |
||||||||||||
by condensation of the amine with benzotriazole and formaldehyde, followed by reaction of the products with Grignard reagents (equation 45)126.
Bt CH2NHR1 C R2MgX ! R2CH2NHR1 |
45 |
1-(Benzotriazol-1-yl)-N-triphenylphosphorylidenemethylamine 125 and lithium amides LiNR1R2 (R1 D Ph, R2 D Me; NR1R2 D pyrrolidin-1-yl or morpholin-4-yl) afford the phosphoranes 126, which are converted into ˛-(arylideneamino)alkylamines 127 on treatment with aromatic aldehydes127.
Bt |
|
CH2 N |
|
PPh3 + LiNR1 R2 |
|
R1 R2 NCH2 N |
|
PPh3 |
|
|
|
|
|||||||
|
|
||||||||
|
|
|
|||||||
(125) |
|
|
(126) |
|
|
||||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
− Ph3 PO |
+ A rCHO |
||
|
|
|
|
|
|
|
|
|
Ar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 R2 NCH2 N |
|||
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
(127) |
|
|||
The N-t-butylation of aromatic amines has been described. The benzotriazoles 128 (R1 D Pr, i-Pr or t-Bu; R2 D Ph, Ar or 2- or 3-pyridyl) react with hydrogen peroxide under

558 |
G. V. Boyd |
selenium dioxide catalysis to yield mixtures of amides 129 and rearranged formamides
130. The latter are formed almost exclusively from 128 (R1 D t-Bu). Hydrolysis yields N-t-butylarylamines128.
R1 CONHR2
(129)
Bt CHR1 NHR2
(128) |
HCONR1 R2 |
for R1 = But |
HNButR2 |
|
(130)
Symmetrical aminals, e.g. 132, are obtained from benzotriazoles such as 131 on treatment with piperidine129.
Bt CHPh N |
N CHPh N |
(131) |
(132) |
Compounds 133, prepared by the condensation of benzotriazole with aldehydes R1CHO (R1 D H, Pr or Ph) and N-octylaminoacetonitrile, react with sodium tetrafluoroborate to give the cyanomethyl derivatives 134. The latter are de-cyanomethylated by the action of copper(II) sulphate. Treatment of 133 with Grignard reagents affords the analogues 135 (R2 D Ph or PhCH2) and thence the secondary amines 136130.
|
|
|
|
|
|
|
CH2 CN |
CuSO4 |
|
|
|
|
|
|
|
|
R1CH2 N |
R1CH2 NHC8 H17 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
|
|
NaBF4 |
|
|
C8 H17 |
|
|
||
|
|
CH2 CN |
|
|
|
|
(134) |
|
|
|
Bt |
CHR1N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
C8 H17 |
|
R2 |
MgX |
CH2 CN |
|
|
|
|
|
|
|
|
|
||||
|
|
(133) |
|
|
|
|
CuSO4 |
|
|
|
|
|
|
|
|
R2 CHR1N |
R2 CHR1NHC8 H17 |
||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
C8 H17 |
|
(136) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(135) |
|
|
|
Primary amides R1CONH |
2 |
(R1 |
D |
Me, Ph etc.), benzotriazole and aldehydes R2CHO |
||||
(R |
2 |
|
|
|
|
|
|
||
D H, Pr, i-Pr, EtO2C etc.) give the benzotriazol-1-yl derivatives 137, which with |
|||||||||
ammonia furnish monoacylated aminals 138131.
Bt CHR2NHCOR1 |
NH3 |
! H2NCHR2NHCOR1 |
|
|
|
(137) |
(138) |
Benzotriazole, secondary amines and ˛,ˇ-unsaturated aldehydes or ketones yield products which exist in solution as equilibrium mixtures of four possible isomers 139 142. Heating this mixture with sodium hydride produces dienamines132.

|
12. Advances in the chemistry of amino and nitro compounds |
559 |
|
1-BtH + |
CHO + HNR2 |
1-Bt 1-Bt |
|
|
|
||
|
Me |
|
|
|
Me |
NR2 |
|
|
|
(139) |
|
|
1-Bt 2-Bt |
|
2-Bt 1-Bt |
|
|
2-Bt 2-Bt |
Me |
NR2 |
Me |
NR2 |
|
Me |
NR2 |
|
(140) |
|
(141) |
|
|
(142) |
 NR2
NR2
1-(Azidomethyl)benzotriazole 143 and triphenylphosphine form the phosphorane 144, which can be converted into diverse primary amines 146 (R1 D C12H25, Ph, PhCH2, PhC C etc.) by treatment with organometallic reagents and hydrolysis of the products 145133. Alkylation of the intermediates 145 with R2I yields secondary amines 147. Other useful transformations of 145 are the formation of carbodiimides 148 by the action of isocyanates and of isothiocyanates 149 with carbon disulphide. Aldehydes give imines 150134.
Bt |
|
CH2 N3 |
|
+ PPh3 |
|
Bt |
|
CH2 N |
|
|
PPh3 |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
− N2 |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
(143) |
|
|
|
|
|
|
(144) |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 CH2 NH2 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
H2 O |
|
|
|
(146) |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
R1M |
|
|
|
|
|
|
1 |
|
2 |
|
|
||||
|
|
|
|
|
|
|
|
|
2 |
I |
R CH2 NHR |
|||||||||||
|
|
|
|
|
|
|
|
|
|
(i) R |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
(ii) NaOH |
(147) |
|
|
|
|
|||||||
|
|
|
R1 CH2 N |
|
PPh3 |
|
R2 NCO |
R1 CH2 N |
|
C |
|
NR2 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
(145) |
|
|
|
|
CS2 |
|
|
(148) |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
R2 CHO |
R1 CH2 NCS |
|||||||||||
|
|
|
|
|
|
|
|
|
|
(149) |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 CH2 N |
|
|
CHR2 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
(150)

560 |
G. V. Boyd |
The N-methylation of primary aromatic amines by means of 1-(hydroxymethyl)-1H- indazole has been described (equation 46)135.
+ A rNH2 |
LiA IH4 |
CH3 NHAr |
N |
N |
|
N |
N |
(46) |
|
||
CH2 OH |
CH2 NHAr |
|
The Mannich reaction of secondary amines R12NH (dibenzylamine, piperidine, morpholine, etc.), aldehydes R2CHO (R2 D alkyl, Ph or 2-furyl) and thiols R3SH (R3 D alkyl, Ph or benzyl) results in ˛-amino sulphides, which react with Grignard compounds to give tertiary amines in good yields (equation 47)136.
R4MgX
R12NH C R2CHO C HSR3 ! R12NCHR2SR3 ! R12NCHR2R4 47
Iminium ions can be generated from tertiary amines and the free radical chlorine dioxide, a gas, which can be stored in aqueous solvents (equation 48)137.
|
ClO2 |
+ |
|
R1R2 N−CHR3 |
ClO2 |
+ |
|
|
R1R2 NCH2 R3 |
|
R1R2 NCH2 R3 |
+ |
|
R1R2 N |
|
CHR3 |
|
|
|
|
−H |
|
|
|
|
|
48
The products can be trapped as ˛-(cyanoalkyl)amines in the presence of sodium cyanide (equation 49)137
Me2 NCH3 + ClO2 |
NaCN |
|
|
|||||
|
|
|
Me2 NCH2 CN |
|
|
|||
|
|
|
|
|||||
Et2 NCH2 Me + ClO2 |
NaCN |
|
|
|||||
|
|
|
|
Et2 NCHCN |
|
|
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
(49) |
|
|
|
|
|
|
|
|
|
|
+ ClO2 |
|
NaCN |
+ |
|
||||
|
|
|
|
|
|
CN |
||
N |
|
|
|
|
N |
N |
||
|
|
|
|
|
||||
CH3 |
|
|
|
|
CH2 CN |
CH3 |
|
|
or internally (equation 50)137.
HO
• ClO2
N
|
HO |
O |
N+ |
− H + |
(50) |
N |
9. By oxidative amination
The Chichibabin reaction138 and other aminations of nitrogen heterocycles139 have been reviewed. Accounts of the introduction of an amino group into azines and nitroaromatic

12. Advances in the chemistry of amino and nitro compounds |
561 |
compounds by means of potassium permanganate in liquid ammonia solution |
have |
appeared140. These reactions proceed by dehydrogenation of intermediate -adducts; see, for example, the formation of 4-amino-3,6-dimethoxypyridazine (151)141.
|
|
|
|
|
H |
NH2 |
NH2 |
||
|
|
|
|
|
|
||||
|
OMe |
|
|
|
OMe |
OMe |
|||
|
|
|
+NH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
−2[H] |
|
|
|
N |
|
|
|
|
N |
|||
|
|
|
|
N |
|||||
MeO |
N |
|
MeO |
N |
|
MeO |
N |
||
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
(151) |
3-Nitropyridine yields |
a mixture of |
2-, |
4- and 6-amino-3-nitropyridines by this |
||||||
method142. An amino group is introduced into the 2-position of 1,n-dinitronaphthalenes (n D 3 8)143 and various 5- and 8-nitroquinolines, such as 8-methyl-5-nitroquinoline and 6-chloro-8-nitroquinoline, have been aminated adjacent to the nitro group144. Pteridines are converted into alkylamino derivatives by the action of a solution of potassium permanganate in an alkylamine, e.g. equation 51145.
|
|
|
|
|
|
NHEt |
|
||
|
|
N |
|
|
|
|
N |
|
|
N |
|
|
|
EtNH2 |
N |
|
|
(51) |
|
|
|
|
|
[O] |
|
|
|
|
|
|
N |
N |
Ph |
|
N |
N |
Ph |
||
10. By photoamination |
|
|
|
|
|
|
|
|
|
Irradiation of |
mixtures of 2-alkoxynaphthalenes (R1 |
D |
Me, Et or i-Bu) and ammonia |
||||||
2 |
(R |
|
|
|
|
|
|
||
or primary amines R NH2 |
D |
Me, Et, Pr, i-Pr or CH2DCHCH2) in aqueous acetonitrile |
|||||||
|
|
|
|
146 |
. |
|
|
|
|
containing m-dicyanobenzene gives adducts 152 |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
NHR2 |
|
|
|
|
|
OR1 |
|
|
|
|
OR1 |
(152)
Photoamination of 7-methoxy-1,2-dihydronaphthalene in the presence of p- dicyanobenzene similarly affords the amine 153147 and 1-benzamido-9,10-anthraquinone reacts with butylamine under UV irradiation in air to yield 154148.
The photoamination of naphthalene, several substituted naphthalenes, anthracene and phenanthrene with ammonia, methylamine or benzylamine in aqueous acetonitrile in the presence of m-dicyanobenzene gave aminated dihydroarenes, e.g. 155. Secondary amines (dimethylamine and diethylamine) react less efficiently149. 9-Methoxyphenanthrene and
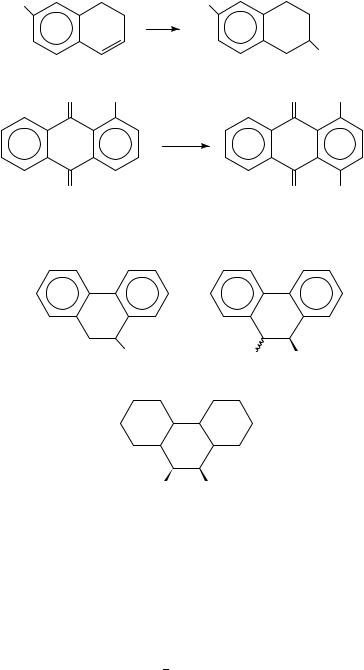
562 |
G. V. Boyd |
MeO |
MeO |
NH2
(153)
O |
NHCOPh |
O |
NHCOPh |
+H2 NBu
−2[H]
O |
O |
NHBu |
(154)
ammonia afforded 156 as a 75:25 mixture of cis- and trans-isomers, whereas with isopropylamine only the cis-adduct 157 was obtained150.
NHEt |
H2 N |
OMe |
|
||
(155) |
|
(156) |
Pri HN |
OMe |
(157)
11. By reductive alkylation
The high-yield reductive methylation of numerous alkyl and arylamines and of dialkyland alkyl-arylamines with paraformaldehyde in the presence of zinc chloride and zinc borohydride has been reported (equation 52)151.
R1R2NH C (HCHO)n ! R1R2NCH3 |
52 |
Refluxing a mixture of an aromatic amine ArNH2 and an aldehyde RCHO (R D Pr, PhCH2, Ph or 4-ClC6H4) in ethanol in the presence of sodium hydrogen telluride gives the alkylated amine ArNHCH2R in 22 95% yields152; secondary aliphatic amines react analogously153.
