

Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups.
Edited by Saul Patai Copyright 1996 John Wiley & Sons, Ltd.
ISBN: 0-471-95171-4
CHAPTER 13
Diazotization of amines and dediazoniation of diazonium ions
HEINRICH ZOLLINGER
Technisch-Chemisches Laboratorium, Eidgenossische¨ Technische Hochschule
(ETH), Zurich,¨ Switzerland
Fax: +41 01 632 1072
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
628 |
II. DIAZOTIZATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
628 |
A. Diazotization with Alkali Nitrite in Aqueous and Concentrated |
|
Mineral Acids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
628 |
B. Isolation of Diazonium Salts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
636 |
C. Diazotization of 2- and 4-Aminophenols . . . . . . . . . . . . . . . . . . . . . |
637 |
D. Formation of Diazonium Salts under Anhydrous Conditions . . . . . . . . |
639 |
E. Mechanism and Kinetics of Rate-limiting Nitrosation Steps . . . . . . . . |
640 |
F. Nucleophilic Catalysis and the Transformation Mechanism |
|
of N-Nitrosoamines into Diazonium Ions . . . . . . . . . . . . . . . . . . . . |
644 |
III. DEDIAZONIATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
646 |
A. Mechanisms of Dediazoniations . . . . . . . . . . . . . . . . . . . . . . . . . . |
646 |
B. Hydro-de-diazoniations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
650 |
C. Halo-de-diazoniations and Related Reactions . . . . . . . . . . . . . . . . . . |
651 |
D. Substitutions of the Diazonio Group by Reagents |
|
with Reacting C-Atoms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
653 |
E. Hydroxyand Mercapto-de-diazoniations . . . . . . . . . . . . . . . . . . . . |
656 |
F. Substitutions of the Diazonio Group by Carbonyl |
|
and Sulfonyl Groups . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
657 |
G. Metallo-de-diazoniations and Related Reactions . . . . . . . . . . . . . . . . |
657 |
H. Photolytic Dediazoniations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
657 |
IV. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
658 |
627

628 |
Heinrich Zollinger |
I. INTRODUCTION
In this chapter diazotization of primary aromatic and heteroaromatic amines and dediazoniation of corresponding diazonium ions are reviewed. In principle, only investigations are included which were not yet mentioned in earlier volumes of The Chemistry of Functional Groups series, i.e. for diazotization the volume on amines1 (1968) and for dediazoniation that on triple-bonded functional groups2 (1983). Exceptions are, of course, earlier investigations which have to be mentioned in the context of papers which were published more recently. Although primary aliphatic amines can be diazotized by the same methods as aromatic amines, the alkanediazonium ions formed are not stable. They lose dinitrogen rapidly and lead to solvolytic and rearranged products. These reactions of alkanediazonium ions were recently reviewed by Zollinger3a.
II.DIAZOTIZATIONS
A.Diazotization with Alkali Nitrite in Aqueous and Concentrated Mineral Acids
This classical method for diazotizations has been in use since the 1860s, in particular for the large-scale production of azo dyes, and was optimized many decades ago. Preparative aspects can be found most conveniently in the books of Fierz-David and Blangey4 and Saunders and Allen5, or in the diazotization chapter of a recent supplementary volume of Houben-Weyl6. There is also a recent review of preparative aspects integrated in physical organic chemistry7a. Godovikova, Rakitin and Khmel’nitzki8 and Zollinger7b reviewed diazotizations of weakly basic amines in strongly acid media. Many diazotizations of specific amines are described in Organic Syntheses. In the indexes of the various volumes of that series they cannot be found, however, under the names of the diazonium salts formed, but in the reaction methods part under ‘Diazotization’ with the name of the final product.
There are some general characteristics of diazotizations which should be mentioned at the beginning of this section. They are related to the amounts of acid and of nitrosating reagents to be used, and to the reaction temperature.
A considerably greater amount of mineral acid than the two equivalents necessary on the basis of the stoichiometry of diazotization should be used even if strongly basic amines are used as reagents. With weakly basic amines the acid concentration has to be even higher in order to dissolve the amine practically completely as ammonium ion.
Compounds bearing both an aliphatic and an aromatic amino group can be selectively diazotized at the aromatic amino group without hydroxy-de-amination at the aliphatic group, as shown by Kornblum and Iffland9 for compounds of type 1. At pH < 3 only the aromatic amino group reacts with the nitrosating reagent; the aliphatic group is much more basic (ca 5 pK units) and so its equilibrium lies much further over in favor of the ammonium form.
H2N |
(CH2)n NH2 |
(1)
In contrast to the acid, sodium nitrite should not in general be added in excess. Firstly, as far as the ratio of amine to nitrite is concerned, diazotization is practically a quantitative reaction. In consequence, it provides the most important method for determining aromatic amines by titration. Secondly, an excess of nitrous acid exerts a very unfavorable influence on the stability of diazo solutions, as was shown by Gies and Pfeil10. Mechanistically the

13. Diazotization of amines and dediazoniation of diazonium ions |
629 |
reactions between aromatic diazonium and nitrite ions were investigated more recently by Opgenorth and Ruchardt¨11. They showed that the primary and major reaction is the formation of aryl radicals from the intermediate 1-aryl-2-nitrodiazene (former name: arenediazonitrite Ar N2 NO2).
It is therefore important to measure the amount of nitrite required for a reaction as exactly as possible. Hence the azo chemist takes the following precautions:
(1)Determination of the content of diazotizable amine by titration with nitrite.
(2)Use of standard solutions of sodium nitrite.
(3)Testing for excess of nitrous acid at the end of the reaction. For this purpose starchpotassium iodide papers are best used, and these indicate nitrite in acid solution by turning blue instantaneously. With some practice, the nitrite reaction can be clearly distinguished from the coloration caused by certain diazo compounds, such as those bearing nitro substituents. The latter react only after 0.5 to 2 seconds. Often the difference becomes more marked after dilution of the diazo solution with concentrated hydrochloric acid. A properly conducted diazotization should exhibit on completion a very weak nitrite
reaction, corresponding to an excess of about 10 4 M.
(4) Nitrite in significant excess has to be destroyed. Traditionally urea has been employed as a nitrous acid scavenger yielding gaseous products, as shown in equation 1, but the reaction is slow. Therefore it was recommended to replace urea by sulfamic acid (equation 2). More recently, Williams and coworkers12 quantitatively compared the reactivity of nine nitrous acid scavengers at various acidities. Their choice of scavengers also included some aromatic amines which, obviously, cannot be used as scavengers for diazotizations, but are suitable for trapping nitrous acid in other systems, e.g. for removing traces of HNO2 from nitric acid. Their results show that, among the scavengers suitable for use in removing excess nitrous acid from diazotization solutions, sulfamic acid has the highest rate at low acidity (0.05 M). Hydrazine and hydrazoic acid are faster at high acidities (0.5 and 1.3 M).
|
NH2 |
|
|
|
OC |
+ 2HNO2 |
CO2 + 2N2 + 3H2 O |
(1) |
|
|
NH2 |
|
|
|
|
OH |
|
|
|
O2S |
+ HNO2 |
|
H2SO4 + N2 + H2O |
(2) |
|
||||
|
NH2 |
|
|
|
A low temperature of diazotization, normally close to 0 °C, is advantageous for two reasons. Firstly, the solubility of free nitrous acid is greater, which means that there is less danger of the nitrous gases escaping from the acid medium. Secondly, the moderate stability of most diazonium salts demands it. These two factors usually outweigh the lower rate of reaction and the poorer solubility of the starting material, which are in themselves undesirable. In cases where the diazo compound is relatively stable, higher temperatures of diazotization may be used, such as 10 15 °C for sulfanilic acid. On a large scale, certain diazotizations are carried out at 30 °C, 40 °C and even higher; for example, 2-amino-5-benzamido-1,10 -diphenyl sulfone and its derivatives, or 3-aminodibenzofuran
(2), are diazotized at 50 °C (see Saunders and Allen5a). Diazotizations should be carried out above room temperature only in cases where a relatively dilute aqueous system (<2 M amine, <1 M mineral acid) is used and the diazonium salt formed does not precipitate.
In general, the temperature is kept at 0 °C most easily by adding ice to the reaction mixture. In this way the considerable heat of reaction evolved during diazotization is dealt with more safely and effectively than by external cooling.

630 |
Heinrich Zollinger |
O |
NH2 |
(2)
The ‘indirect’ method of diazotization is often used for aminoarenesulfonic acids which are relatively insoluble in acid solution where they are present as zwitterions (3 in the case of sulfanilic acid; see equation 3). The easily soluble anion 4 is obtained by introducing the required amount of sodium carbonate or hydroxide, and nitrite is added to the approximately neutral solution, which is then run into mineral acid. Indirect diazotization is particularly recommended for the aminosulfonic acids of greater molecular mass but, contrary to some statements in the literature, the three anilinesulfonic acids themselves can be diazotized directly in suspension, the reaction proceeding quite smoothly after some practice.
+ |
SO3 − |
|
+ OH− |
SO3 − |
(3) |
||
H3 N |
|
|
|
H2 N |
|||
|
|
||||||
|
|
|
+ H+ |
|
|
||
(3) |
|
|
|
|
|
(4) |
|
The diazotization of heteroaromatic amines is a ticklish procedure. In spite of the great increase in interest for disperse dyes based on heterocyclic diazo components, little systematic knowledge is available. In a review of such diazo components13 practically nothing is mentioned on suitable methods of diazotization and on yields (which are in part low). The somewhat older review of Butler14 is, in this respect, more informative. So too is the section on synthesis in the general review on diazoazoles by Cirrincione and coworkers15.
The diazotization of heteroaromatic amines is basically analogous to that of aromatic amines. Among the five-membered systems the amino-azoles (pyrroles, diazoles, triazoles, tetrazoles, oxazoles, isooxazoles, thia-, selenaand dithiazoles) have all been diazotized. In general, diazotization in dilute mineral acid is possible, but diazotization in concentrated sulfuric acid (nitrosylsulfuric acid, see below) or in organic solvents using an ester of nitrous acid (ethyl or 2-pentyl nitrite) is often preferable. Amino derivatives of aromatic heterocycles without ring nitrogen (furan and thiophene) can also be diazotized.
A characteristic property of most diazotizations of aminoazoles is the occurrence of a relatively stable transient intermediate (probably the N-nitrosoamine), in contrast with the diazotization of carbocyclic aromatic amines, where N-nitrosoamines have been considered to be unstable intermediates.
For the diazotization of heteroaromatic amines of the azole type, it is important to be aware of the fact that the heterocyclic nitrogen (or one of them in di-, triand tetrazoles) is more basic than the amino nitrogen. As a consequence the first diazonium ions formed may react with the starting material still present, forming a triazene. This secondary reaction can be avoided by working in a more acidic medium. For example, as shown in Scheme 1, diazotization of 3-amino-1H-pyrazole (5) yields pyrazole-3-diazonium salts (6) only in strong acids, e.g. 75% phosphoric acid; in aqueous acetic acid the product is the 1,3- di[30,300 -pyrazolo]-triazene (8) as found by Reimlinger and coworkers16. The diazonium chloride (6, X D Cl) can be isolated by precipitation with ether if the diazotization is carried out with 2-pentyl nitrite in methanol saturated with HCl gas. Dissolving the salt in chloroform leads to deprotonation, giving the (mesomeric) diazoalkane 7. This compound
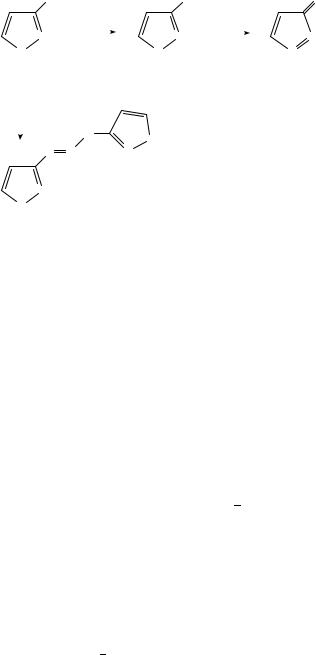
13. Diazotization of amines and dediazoniation of diazonium ions |
631 |
|||||||
|
NH2 |
|
|
N2+ |
X− |
N2 |
|
|
|
|
|
|
|
|
|
||
|
N |
|
HONO |
N |
|
CHCl3 |
N |
|
|
|
strongacid |
|
+ |
|
|||
N |
|
|
N |
|
−H , −X |
N |
|
|
|
|
|
|
|
||||
H |
|
|
H |
|
|
|
|
|
(5) |
|
|
(6) |
|
|
(7) |
|
|
|
|
|
|
|
|
|
|
|
HONO |
HOAc |
|
|
|
|
|
|
|
|
|
|
HN |
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
N |
N |
|
|
|
|
|
N
N
H
(8)
SCHEME 1
shows an infrared NN band (2130 cm 1) comparable to those of diazoalkanes, in contrast to the diazonium band of the cation (2205 cm 1).
The diazonium ions formed from aminoazoles are relatively strong acids. The pKa values of five di-, triand tetrazolediazonium ions are reported to be between 3 and 4, i.e. about 10 units lower (more acidic) than those of the respective unsubstituted heterocycles (Vilarrasa and coworkers17). Therefore, deprotonation of the diazonium ion is easy and, depending on reaction conditions, yields either the diazonium salt or its conjugate base, i.e. the diazo compound. The electrophilic reactivity of the ˇ nitrogen atom in the diazo group of the base is lower than the reactivity of the diazonio group of the cation18.
Reactions between 3-amino-2-pyrazolines (9) and nitrous acid were studied by Gorelik’s group. The unsubstituted 3-amino-2-pyrazoline (9, R D H) forms the pyrazole-3- diazonium ion (11, R D H) in a combined hydrogenation and diazotization by the direct action of the diazotizing agent (2 equivalents) on the dihydroaromatic amine (Scheme 2, pathway A, Gorelik and Lomzakova19). For 1-phenyl-3-amino-2-pyrazoline (9, R D C6H5) a completely different reaction was found (equation 5, pathway B). When aqueous NaNO2 is added to a solution of 9 (R D C6H5) in dilute HCl, the red color characteristic of the cation radical 10 is observed. After 1 2 min it changes into a blue color. The blue compound when isolated corresponds to the dichloride of the dication of 12. If the order in which these reagents are mixed is changed, the reaction leads to a different result. A solution of 9 (R D C6H5) and two equivalents of NaNO2 in aqueous acetone is added to 17% HCl/H2O over 1.5 hours. The product is 1-phenylpyrazole-3- diazonium chloride (11, R D C6H5; Gorelik and coworkers20). This reaction therefore corresponds to pathway A obtained with 3-amino-2-pyrazoline.
However, Gorelik’s group20 found even a fourth alternative reaction! If the phenyl group in 1-phenyl-3-amino-2-pyrazoline contains electron-withdrawing substituents (2,4-dinitro or 4-phenylsulfonyl), diazotization is faster than dehydrogenation and consequently the 1-aryl-2-pyrazoline-3-diazonium ions 13 are formed (pathway C). Evidently the higher oxidation reduction potential is increased by these substituents and prevents their oxidation to cation-radicals of the type 10 [R D 2,4-(NO2)2C6H3, 4-CH3COC6H4 or 4-C6H5SO2C6H4].

632 |
|
|
|
|
Heinrich Zollinger |
|
|
|
|
||
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
N |
N |
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
HNO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
(9) |
|
C |
R |
|
|
|
+ |
|
N |
|
|
|
|
N |
N |
|
|
|
N |
|
|
|
|
|
|
|
|||
|
|
|
|
NH |
|
|
|
|
|
N |
+ |
|
|
|
|
2 |
|
|
|
|
|
2 |
|
|
(10) |
|
|
B |
|
|
|
|
(13) |
|
|
|
A |
|
(R = C6 H5) |
|
|
|
|
|
|
||
R |
HNO2 |
|
|
|
|
|
|
|
|
||
N |
N |
|
|
N |
N |
|
|
|
N |
N |
|
|
N |
+ |
H |
N |
|
|
|
|
|
NH |
|
|
2 |
2 |
|
|
|
|
|
|
2 |
||
|
(11) |
|
|
|
|
|
[O] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
+ |
|
|
|
+ |
N |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
N |
|
|
|
N |
|
||
|
|
|
H2 N |
|
(12) |
|
|
NH2 |
|||
|
|
|
|
|
|
|
|
|
|
||
R= H or aryl(see text)
SCHEME 2
The diazonium ions 13 with electron-withdrawing substituents are not heteroaromatic compounds and therefore do not strictly come within the scope of this chapter. They are formally related to the alkenediazonium ions. Nevertheless, they are discussed here because in their properties they bear a close resemblance to heteroaromatic and arenediazonium ions rather than to alkenediazonium ions. In particular they can be obtained by direct diazotization of the amines, they are stable in an aqueous medium and they are capable of undergoing an azo coupling reaction.
In some cases the primary diazotization products cannot be isolated. For example, diazotization of 2-methyl-5-aminotetrazole (14) directly yields the triazene 15, i.e. the N-coupling product, since the intermediate diazonium ion is reactive enough to give the N- coupling product with the parent amine even under strongly acidic conditions (equation 4; Butler and Scott21).
H3 C |
|
|
H3 C |
|
|
|
|
N |
N |
N |
N |
|
|
CH3 |
|
2 |
NH2 |
HONO |
|
|
N |
N |
N |
H+ |
|
|
N NH |
(4) |
|||
|
|
|
|
|
|||
N |
N |
N |
N |
|
|
||
|
|
|
|||||
|
|
|
|
|
|
N |
N |
|
(14) |
|
|
|
|
(15) |
|

13. Diazotization of amines and dediazoniation of diazonium ions |
633 |
5-Diazotetrazole (16) was obtained by dropwise addition of 2-pentyl nitrite to a solution of 5-amino-1H-tetrazole in a 4:1 mixture of tetrahydrofuran and aqueous hydrochloric acid. The diazonium chloride can be extracted into ether. Shevlin22 obtained the extremely explosive solid diazonium salt (16) by evaporation of that solution. He has recommended that not more than 0.75 mmol of diazonium salt be isolated at one time. An explosion during the diazotization of 5-aminotetrazole on a laboratory scale was described by Gray and coworkers23. The structure 17 (equation 5) indicates clearly that this diazo compound may have the tendency to decompose into ‘atomic carbon’ and three equivalents of dinitrogen a reaction which is clearly highly exothermic. The decomposition of the tetrazole-5-diazonium chloride (16) has been studied by Shevlin22 by coating the salt on the walls of a 500 ml flask in the presence of two substrates, ethene and ethylene oxide. With ethene the products found after heating the flask to 80 °C are shown in equation 6, and with ethylene oxide in equation 7. The products correspond to those found with atomic carbon formed by completely different methods (see references cited by Shevlin).
N |
N |
|
|
N |
N |
|
|
|
+ |
− H+ |
|
|
|
|
|
N2 |
|
|
N2 |
(5) |
|
|
− X− |
||||
|
|
X− |
|
|
||
N |
N |
|
N |
N |
|
|
|
|
|
||||
H
(16) |
(17) |
+H2 C  CH2
CH2
16
+ H2 C CH2
O
N2 + CH4 + H2 C C
C CH2 + CH3 C CH + HCN
CH2 + CH3 C CH + HCN
N2 + CO + H2 C CH2 + CH4 + HC
CH2 + CH4 + HC CH
CH
(6)
(7)
Diazotization of the aminopyridines and aminopyridine oxides was studied in detail by Kalatzis and coworkers. Diazotization of 3-aminopyridine and its derivatives is similar to that of aromatic amines because of the formation of rather stable diazonium ions. 2- and 4-aminopyridines were considered to resist diazotization or to form mainly the corresponding hydroxy compounds. However, Kalatzis24 showed that true diazotization of these compounds proceeds in a similar way to that of the aromatic amines in 0.5 4.0 M hydrochloric, sulfuric or perchloric acid, by mixing the solutions with aqueous sodium nitrite at 0 °C. However, the rapidly formed diazonium ion is hydrolyzed very easily within a few minutes (hydroxy-de-diazoniation). The diazonium ion must be used immediately after formation, e.g. for a diazo coupling reaction, or must be stabilized as the diazoate by prompt neutralization (after 45 s) to pH 10 11 with sodium hydroxide borax buffer. All isomeric aminopyridine-1-oxides can be diazotized in the usual way (Kalatzis and Mastrokalos25). The diazotization of 5-aminopyrimidines results in a complex ring opening and conversion into other heterocyclic systems (see Nemeryuk and coworkers26).
When the heteroaromatic amine is insufficiently soluble in aqueous acid, it can be dissolved in the minimum volume of an organic solvent miscible with water. Dilute mineral acid and a solution of sodium nitrite are then added. An example is the diazotization of 2-phenyl-3,4-acetyl-5-methyl-pyrrole (Dattolo and coworkers27). As the amines become

634 |
Heinrich Zollinger |
more weakly basic, the normal method of diazotization becomes progressively more difficult. The equilibrium between amine and ammonium salt increasingly favors the former which, usually because of its poor solubility in water, is prevented from taking part in the reaction. Research into the mechanism of diazotization has demonstrated that the important step is the addition of the nitrosating agent to the base of the amine. Thus, the acidity for each diazotization should be so chosen that the equilibrium concentration of base corresponds to that of its saturated solution. This rule leads to the use of higher concentrations of aqueous mineral acid for weakly basic amines.
Yet in more concentrated aqueous mineral acids additional complications have to be considered. In hydrochloric acid containing more than 20% HCl, nitrous acid begins to exert an unfavorable oxidizing effect according to equation 8. As Hoffman28 showed in a contribution to Organic Syntheses, diazotizations can be performed in mixtures of concentrated aqueous hydrochloric acid and glacial acetic acid (10:3). When nitrite solution is added dropwise to sulfuric or nitric acid of concentration greater than 25%, the rate of evolution of nitrous gases is greater than that of nitrosation.
2NO2 C 2Cl C 4HC ! 2NO C Cl2 C 2H2O 8
Diazotization can be carried out without difficulty in 90 96% sulfuric acid, however. Nitrous fumes are given off as soon as aqueous solutions of nitrite salts are added to sulfuric acid of lower concentration, but solid sodium nitrite can be dissolved in 90 96% sulfuric acid at 0 10 °C smoothly and without evolution of gas. Nitrosylsulfuric acid, NOC HSO4 , is formed. Directions for the preparation of 2 M nitrosylsulfuric acid are given by Fierz-David and Blangey4a, but sodium hydrogen sulfate crystallizes after some time from acid of this strength so that it is best to prepare a stock solution of 1 M sodium nitrite in 96% sulfuric acid, which is quite stable at room temperature.
More concentrated solutions of nitrosylsulfuric acid containing no sodium ions can be obtained by reducing a solution of nitric acid in concentrated sulfuric acid with gaseous SO2.
We shall discuss the acid base equilibria of nitrous acid in aqueous mineral acids of increasing concentration in Section II.E.
The nitrosylsulfuric acid method is particularly suitable for the diazotization of diand trinitroanilines and aminoanthraquinones. Such amines may be added directly to the nitrosylsulfuric acid, but it is preferable to run the appropriate amount of nitrosylsulfuric acid into a solution of the amine in 96% sulfuric acid. In general, these diazotizations can be carried out at room temperature. The end-point is determined in the usual manner with iodide paper, but only after first diluting a few drops with ice. On completion, the whole is diluted with ice. The test with iodide paper fails in the case of polynitrodiazonium salts.
In 1969 a serious explosion took place in Basle when 287 kg (1.3 kmol) of 2-chloro- 4,6-dinitroaniline was diazotized in 384 kg 40% nitrosylsulfuric acid. The temperature was increased from 30 °C to 50 °C and kept at that level. Shortly afterwards the explosion occurred; three workers were killed and 31 injured, some seriously. The reaction had been carried out twice before in the same way without difficulty. Detailed investigations (Bersier, Valpiana and Zubler29) with the help of differential scanning calorimetry showed that, at the high concentration of that batch, a strongly exothermic reaction (1500 kJ/kg) starts at about 77 °C. In contrast, when the reactants were diluted with 96% sulfuric acid to twice the volume, the reaction was found to begin at 146 °C, generating only 200 kJ/kg.
Bersier and coworkers29 published a list of 20 aromatic and heteroaromatic amines whose stabilities in diazotization systems have been investigated. Aqueous systems are harmless, even with amines containing one or two nitro groups (provided that they can be diazotized at all in water). In 96% sulfuric acid, diazotizations of aminoanthraquinones are not dangerous; this is also the case for heteroaromatic amines in mixtures of sulfuric acid
13. Diazotization of amines and dediazoniation of diazonium ions |
635 |
and acetic acid. Diazotization of dinitroand halogenodinitroanilines in 96% sulfuric acid, particularly with amine concentrations above 1 mol/kg, is dangerous. Aqueous systems are less hazardous because of the higher specific heat of water relative to that of sulfuric acid. If diazonium salts precipitate during the reaction one has to be careful in all cases, as solid diazonium salts can detonate (see Section II.B).
If an aromatic o-diamine such as 1,2-diaminobenzene (18) is diazotized in dilute aqueous acid, the 2-aminobenzene-1-diazonium ion formed first (19) undergoes a rapid intramolecular N-azo coupling reaction to give 1,2,3-benzotriazole (20). Both amino groups of 18 can, however, be diazotized in concentrated acid (Scheme 3), forming the bisdiazonium ion 21. 1,3- and 1,4-diamines must also be bisdiazotized in concentrated acids in order to avoid intermolecular N- or C-coupling.
NH2 |
N2+ |
HNO2 |
|
NH2 |
NH2 |
(18) |
(19) |
dilute acids |
|
−H+ |
HNO2 conc.acids |
N |
N2+ |
N |
|
N |
N2+ |
H |
|
(20) |
(21) |
SCHEME 3
If the water content of the diazotization system is too high, the halogen atom in halogensubstituted monoand dinitroanilines may be replaced by a hydroxy group in a bimolecular aromatic substitution. Analogous behavior was observed by Stacey’s group30 in the diazotization of pentafluoroaniline, where the 4-fluoro substituent became hydrolyzed. Later, Sonoda and Kobayashi’s group31 found that this side reaction does not take place if the diazotization is conducted in a dichloromethane aqueous sulfuric acid two-phase system in the presence of tetrakis[3,5-bis(trifluoromethyl)-phenyl]borate.
In some cases the use of nitrosylsulfuric acid may be avoided if 1-naphthalenesulfonic acid is added to moderately concentrated sulfuric acid (20 60%). This greatly reduces the evolution of nitrous fumes compared with a solution of pure sulfuric acid of the same hydrogen ion concentration. It has not yet been investigated whether the phenomenon is due to the formation of an ion pair, [C10H7SO3 NOC], or whether it is simply a solubility effect. In any case, the total acidity range of 4 12 M has thereby become available for diazotization; technically crude sulfonation mixtures are used after dilution with water, for example, a solution of total acidity 4 M of which 2.7 M is due to sulfuric acid. A further advantage of the method lies in the stabilizing effect of the naphthalenesulfonic acid on the diazonium compounds formed (see Section II.B).
The rates of diazotizations in nitrosylsulfuric acid can be increased favorably by the addition of acetic or propionic acid. A mixture of the two acids is frequently used as an additive in diazotizations of heteroaromatic amines, as it has a lower melting point than acetic acid (0 °C or lower), but little is mentioned about it in the scientific literature

636 |
Heinrich Zollinger |
(review: Butler14). A good example of a diazotization in an acetic/propionic acid mixture at 40 °C was described by Goerdeler and Roegler32 for 3-amino-5-phenylisothiazole (22). In phosphoric acid, in contrast, a diazotization of this compound gave a very low yield in the azo coupling reaction. The diazonium salts of the isomeric 5-amino- isothiazoles can be obtained by diazotization in 2 M H2SO4, but they are not very stable, except when they contain electron-attracting substituents (CN, COCH3 or COOEt) in the 4-position. Other examples of diazotizations in acetic/propionic acid mixtures have been described in detail by Ginsberg and Goerdeler and by Alty and coworkers33,34.
NH2
C6H5 N
S
(22)
Belyaev and coworkers35 demonstrated that weakly basic aromatic amines which have a low solubility in diazotizing systems can be diazotized smoothly and with excellent yields (>97%) in mixtures of acetic acid and polyphosphoric acid.
B. Isolation of Diazonium Salts
In most cases diazonium salts are not isolated, but are converted into products by reactions that can be carried out in situ. Moreover, it is actually recommended not to isolate these salts, not even for purification purposes, as many of them have a tendency to explode. In addition, the high solubility of most diazonium salts in water makes precipitation from this medium difficult. Therefore, to obtain solid diazonium salts the recommended method for many decades was to carry out diazotizations in ethanol followed by precipitation with ether. As inorganic salts of nitrous acid are scarcely soluble in ethanol, Knoevenagel recommended alkyl nitrites (ethyl or 2-pentyl nitrite) as diazotization reagents as long ago as 1890. Various other solvents have subsequently been used for diazotizations with alkyl nitrites (see Saunders and Allen5b), but as a method for obtaining solid diazonium salts this has been superseded by the isolation of diazonium tetrafluoroborates and, to a lesser degree, of hexafluorophosphates.
These salts can be made easily since tetrafluroboric acid (HBF4) and hexafluorophosphoric acid (HPF6) are commercially available. However, the main advantage of the diazonium salts with the anions of these acids is their stability, which is significantly higher than that of probably all other diazonium salts. 4-Nitrobenzenediazonium tetrafluoroborate is nowadays even a commercial product. Preparative diazotization methods with these two acids can be found in Organic Syntheses (tetrafluoroborate: Starkey36; hexafluorophosphate: Rutherford and Redmont37).
Another group of stable diazonium salts are the so-called diazonium metal double salts: the zinc double chlorides are particularly important. The term is misleading, as they are not associations between two salts but, in the case of Zn, are formed from two arenediazonium ions with the complex anion ZnCl42 . This fact became obvious from crystal structure studies38 41. The reason for their increased stability relative to ArN2C Cl salts is that ZnCl42 complex ions are less nucleophilic than free chloride ions.
Salts of diazonium ions with certain arenesulfonate ions also have a relatively high stability in the solid state. They are also used for inhibiting the decomposition of diazonium ions in solution. Experimental data42,43 point to the formation of molecular
