

13. Photochemistry of compounds containing CDC double bonds |
681 |
||||
|
|
|
|
|
OTMS |
|
TMSO |
|
|
|
|
|
+ |
hν |
|
||
|
|
H |
OTMS |
||
|
TMSO |
|
|||
O |
|
|
O |
|
|
(213) |
(214) |
(215) |
|
||
(−)-piperitone |
|
|
|
∆ |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
OTMS
OTMS
|
O |
(217) (+)-daucene |
(216) |
TMSO = trimethylsiloxy |
|
SCHEME 46
The first enantiomerically pure synthesis of the antitumor compound quadrone has been developed by Smith and coworkers103, via photoaddition of isobutylene to 218 followed by epimerization affording the desired photoproduct 219˛ in 5:1 ratio with its diastereomer in 74% yield. The synthesis of (C)-enantioquadrone was completed via kinetic resolution of 219˛ (Scheme 47).
O |
|
|
O |
H |
|
|
|
|
|
|
|
|
+ |
hν |
|
O |
|
|
|
|
|
||
|
|
α : β = 5 : 1 |
|
H |
H |
CO |
Me |
CO |
Me |
|
|
2 |
|
|
2 |
|
O |
|
|
|
|
O |
|
(218) |
|
(219) |
|
(220) (+) enantioquadrone |
|
SCHEME 47
Recently, the same group104 has reported the first total synthesis of the novel antitumor metabolite ( )-echinosporin 224. The synthesis is based on an asymmetric [2 C 2] photocycloaddition, which constitutes the cornerstone of the synthetic strategy (Scheme 48).
The presented examples on the synthetic utility of the discussed reaction certainly do not include all the cases which reported in the recent literature because of space limitation. However, excellent reviews54 58 can be consulted in this regard.

682 |
|
|
Nizar Haddad |
|
|
|
|
O |
H |
|
|
O |
|
|
|
|
|
|
H |
|
|
||
|
|
|
|
|
|
||
|
|
O |
|
|
|
|
|
|
|
hν |
|
H |
O |
|
|
|
|
|
|
|
|
||
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
|
|
|
|
O |
|
H |
|
|
H |
|
|
|
|
|
|
O |
H |
|
||
|
|
|
|
|
|
||
|
(221) |
|
|
|
(222) 50% |
|
|
|
|
CO2 Me |
|
|
|
O |
|
|
HO |
|
|
HO |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONH2 |
|
|
|
CONH |
|
|
|
|
|
|
|
2 |
|
|
H |
O |
|
|
H |
O |
|
|
|
|
|
|
||
|
|
|
|
|
H |
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(223) |
|
|
(224) (−)-echinosporin (XK-213) |
||
SCHEME 48
2. Intramolecular photocycloadditions
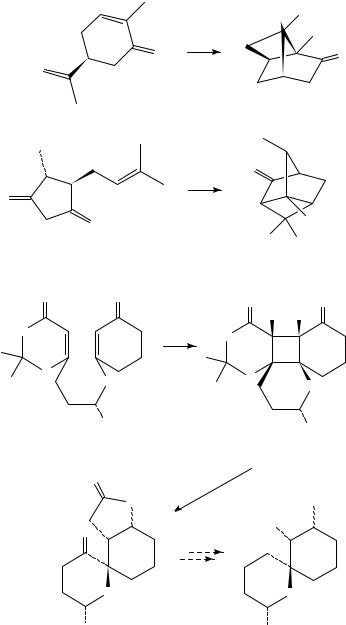
The stereoselectivity in the intramolecular photocycloadditions of mixed alkenes can be controlled to some extent by geometrical constraints. The effect of substituents and chain length in the reactants has been extensively investigated in the photoaddition of alkenes to enones. In many cases, the selectivity can be predicted on rigid structures possessing the reacting alkene at an appropriate distance. For instance, the photocyclization of cage compounds 225 229 is used as the key step in the synthesis of cubane105 230, pentaprismane106 231, hexaprismane skeleton107 232, and other highly strained compounds108 110 (Scheme 49).
Demonstration of the unique synthetic utility of the [2 C 2] photocycloaddition reaction of enones to alkenes and the success in controlling the stereoselectivity, to some extent, in the intermolecular additions (discussed above) prompted further studies and development of new synthetic applications in the intramolecular photoadditions during the last decade. In most cases that have been studied, the alkene was tethered to the cyclic enone by three carbon units or two carbons and one heteroatom.
High stereoselectivity was found in the first example of intramolecular [2 C 2] photocycloaddition discovered by Ciamician and Silber111 in the irradiation of camphor 233 to carvon camphor 234. Generally, if the tethered alkenyl side chain is connected to the enone via an asymmetric center, the configuration of this center plays an important role on the diastereofacial selectivity. For example, high stereoselectivity was found in the irradiation of the diketone 235 or its corresponding enol acetates112, when substituents on the alkenyl side chain affect the selectivity of the enolization of 235 but not the diastereofacial selectivity (Scheme 50).
One of the first examples of high asymmetric induction in the intramolecular [2 C 2] photocycloaddition in which the chiral center located at the side chain found in Winkler and coworkers113 approach to the synthesis of ( )-histrionicotoxin alkaloid 240. Irradiation of 237 is the key step in the synthetic strategy. The isomer 238 was formed in

13. Photochemistry of compounds containing CDC double bonds |
683 |
||
O |
Br |
O |
|
|
|
||
Br |
|
|
|
1. hν |
|
|
|
MeOH, HCl |
|
|
|
2. H2 O |
Br |
|
|
|
|
Br |
O |
|
|
|
|
(225) |
O |
|
(230) cubane |
|
|
|
|
MeO OMe |
|
O |
|
|
|
1. hν |
|
|
|
2. H+ |
|
(226) |
|
|
(231) pentaprismane |
|
O |
|
O |
O |
|
|
O |
|
|
|
hν |
(227) |
|
|
(232) hexaprismane |
|
|
|
|
skeleton |
|
O |
O |
O |
O |
|
|
||||
|
MeO |
|||
O |
O |
MeO |
||
hν |
|
|
hν |
|
O |
O |
MeO |
|
|
O |
O |
MeO |
||
|
||||
(228) |
Reference 108 |
(229) |
Reference 109 |
|
|
SCHEME 49 |
|
|
quantitative yield and was then transformed to the corresponding spirostructure 239 upon reduction with NaBH4 (Scheme 51).
Another example is Crimmins and Gould’s114 total synthesis of (š)-laurenene 243 via the intramolecular photoaddition of enone 241, affording an epimeric mixture with very high facial selectivity, followed by subsequent transformations (Scheme 52).
684 |
Nizar Haddad |
|
|
|
hν |
|
O |
|
O |
|
|
|
(233) |
|
(234) |
|
|
O |
|
|
hν |
|
|
O |
|
|
|
|
O |
|
OAc |
|
|
|
|
|
(235) |
|
(236) |
|
SCHEME 50 |
|
|
O |
O |
O |
O |
|
|
||
|
|
|
H H |
O |
hν |
O |
|
|
|
||
O |
NH |
O |
|
|
NH |
||
|
|
||
|
|
|
|
|
COOMe |
|
CO2 Me |
|
|
|
|
|
(237) |
|
(238) |
|
|
|
|
|
O |
|
|
|
O |
|
HO |
|
O |
|
R |
|
|
|
NH |
NH |
CO2 Me |
R′ |
|
histrionicotoxin |
(239) |
(240) |
SCHEME 51

13. Photochemistry of compounds containing CDC double bonds |
685 |
CO2 Me
CO2 Me
hν
O
O
(241) |
(242) |
H H
H
laurenene
(243)
SCHEME 52
O
|
O |
O |
O |
hν |
|
|
H |
(244) |
(245) |
O |
O |
H |
H
O
hν O
H
H
(247)
(246)
O
H O
H
O
hν O
H
H
(249)
(248)
SCHEME 53

686 Nizar Haddad
Very high diastereofacial selectivity was obtained in the intramolecular photoadditions of the chiral unsaturated lactones115 244, 246 and 248 (Scheme 53).
Koga’s group described the first example of an asymmetric total synthesis of stoechospermol using the photoaddition of 250 as the key step in the synthetic sequence. Irradiation followed by deprotection of the silyl ether and subsequent oxidation afforded the single product 251, indicating the high regioand stereoselectivity of the photocycloaddition116 (Scheme 54).
O |
O |
O |
|
O |
H |
|
|||
|
|
|
|
|
|
|
hν |
|
|
O |
|
O |
|
|
|
|
H |
H |
O |
O |
OTBDMS |
O |
|
|
|
|
|||
(250) |
|
(251) |
|
|
OH
H
H H
Stoechospermol
SCHEME 54
Winkler and coworkers117 have elegantly applied the intramolecular photoaddition of the tricyclic dioxinone photosubstrate 252 in the first synthesis of the ingenane tricyclic ring system 254. The work has recently been extended to the preparation of ingenane 255, the first analog of ingenol 256 to have high affinity for protein kinase C118 (Scheme 55).
Another successful application of the photoaddition fragmentation strategy of dioxinones, with complete stereoselectivity, defined by the configuration of the stereogenic center connecting the alkenyl side chain and by the orientation of the alkene in the first bond-forming step, was found in the photoaddition of dioxinone 257, which afforded the single diastereomer 258. Fragmentation under basic conditions gave the cis-bridged ketoester 259 with the desired configuration at the stereogenic centers as found in the target molecule taxol 260119 (Scheme 56).
All the above examples share high stereofacial selectivity defined by the configuration of the stereogenic center that connects the enone chromophore with the alkenyl side chain. However, chiral induction at the enone and/or the alkenyl tethered must be introduced to achieve stereofacial selectivity in the more general systems in which the alkene is connected at the ˛-carbon or ˇ-carbon of the enone. One of the successful early examples is found in Pirrung’s120 synthesis of (š)-isocomene 263. Irradiation of 261 afforded the single product 262, which was transformed to isocomene in a two-step sequence.

13. Photochemistry of compounds containing CDC double bonds |
687 |
|||
O |
|
H |
O |
|
O |
hν |
|
|
O |
O |
|
|
|
O |
H |
|
H |
|
|
(252) |
|
(253) |
|
|
O |
|
|
|
|
|
H |
|
|
|
H |
|
|
|
|
|
CO2 H |
|
H |
|
(254) |
|
|
|
|
|
|
|
|
|
O |
|
O |
H |
|
|
|
|
|
|
H |
|
|
H |
|
PhCOO H |
|
HO HO |
|
|
CH2 OH |
|
HO |
CH2 OH |
|
(255) |
|
(256) ingenol |
|
|
SCHEME 55 |
|
|
|
|
Minor structural variations were found to affect the stereoselectivity of the photoaddition. Irradiation of 264 under similar conditions afforded a mixture of four isomers and the facial selectivity was reduced to 3:1 (Scheme 57).
Similar diastereofacial selectivity was found in the photoaddition of 267 affording a mixture of 268 and 269 in 4:1 ratio respectively. This reaction was used as the key step reaction in the synthesis of (š)-cedrene 269121 (Scheme 58).
The effect of substituents at the ˛-carbon of the enone on the stereoselectivity was examined on compounds 270, with the stereogenic center located at the alkenyl side chain. Crimmins and DeLoach122 found that the stereoselectivity encountered upon irradiation of 270 depends on the degree of steric hindrance associated with the ester group linked to the double bond. The ratio of the two epimeric centers at the C-9 position varied from 13:1 (R D Me) to 17:1 (R D Et), then 20:1 (R D i-Pr). These results demonstrate that steric effects play an important role in controlling the stereofacial selectivity in these and related systems. Fragmentation of the photoproduced four-membered ring and simple transformations afforded synthesis of (š)-pentalene 274, (š)-pentalenic acid 275 and (š)-deoxypentalenic acid 276 (Scheme 59).
High facial selectivity was also achieved in systems with the alkenyl side chain at the ˛-carbon of the enone. Irradiation of 277 afforded a single stereoisomer 278 in 98% yield, which was used as a key compound in the synthesis of (š)-epi-precapnelladiene123 279.

688 |
|
|
|
Nizar Haddad |
|
|
||
O |
O |
|
|
|
|
O |
|
O |
|
|
|
|
|
|
H |
|
|
O |
|
|
|
|
hν |
O |
|
|
|
|
|
|
|
|
|
|
|
H |
|
H |
|
OMe |
|
|
|
OMe |
|
|
|
|
|
H |
H |
||
|
(257) |
|
|
|
|
|
|
(258) |
|
H |
|
|
O |
|
|
|
|
|
|
CO2 Me |
|
|
|
|
|
|
|
H |
|
H |
|
OMe |
|
|
|
|
|
|
(259) |
|
|
|
|
|
|
|
|
|
|
|
AcO |
|
|
|
|
|
|
|
|
|
O |
|
|
O |
Ph |
O |
|
|
|
OH |
|
Ph |
N |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
OH |
|
|
|
|
|
|
|
|
|
|
HO |
|
H |
O |
|
|
|
|
(260) |
PhCOO |
AcO |
|
|
|
|
|
|
|
taxol |
|
|
|
|
|
|
|
|
|
|
|
|
SCHEME 56
SCHEME 57

13. Photochemistry of compounds containing CDC double bonds |
689 |
SCHEME 58
O |
CO2 Et |
O |
CO2 R |
|
|
O |
CO2 R |
|
|
|
|
CO2 Et |
|
CO2 Et |
|||
|
CO2 R |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
hν |
|
|
H |
+ |
|
|
H |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
|
(270) |
|
(271) |
|
|
|
(272) |
|
|
|
(a) R= Me |
13 |
|
: |
1 |
|
|
|
|
(b) R= Et |
17 |
|
: |
1 |
Li / NH3 |
|
|
|
(c) R= i-Pr |
20 |
|
: |
1 |
|
|
HO
CO2 R |
CO2 Et |
|
|
|
H |
|
|
(273) |
H |
HO |
H |
H |
||
|
CO2 H |
CO2 H |
(274) |
(275) |
(276) |
pentalene |
pentalenic acid |
deoxypentalenic acid |
|
SCHEME 59 |
|

690 |
Nizar Haddad |
The high stereoselectivity was attributed to a repulsive interaction between the methyl group on the side chain and the benzoate enol ether group. Unfortunately, the extremely high stereoselectivity obtained in this system is rather exceptional as could be seen from the irradiation of the related compound 280, elegantly used as the key reaction in a synthesis of ˇ-bulnesene124 282 (Scheme 60).
O O 
hν
OCOPh
(277)
O
hν
OAc
(280)
O
hν
R1
R2 R3
(283)
(a)R1 = t-Bu, R2 = R3 = Me
(b)R1 = t-Bu, R2 = Me, R3 = H
(c)R1 = t-Bu, R2 = R3 = CH2 (allene)
(d)R1 = t-Bu, R2 = R3 = H
(e)R1 = R2 = R3 = Me
OCOPh |
|
|
H |
|
|
Reference |
|
(278) |
|
(279) |
|
|
123 |
||
|
|
epi-precapnelladiene |
|
O |
|
|
|
|
H |
|
|
OCOPh |
|
|
Reference |
|
|
|
|
|
|
|
124 |
(281) |
|
(282) |
|
β-Me / α-Me =3.3 |
|
β-bulnesene |
|
SCHEME 60 |
|
|
|
O |
|
O |
|
|
R3 |
+ |
R3 |
|
|
||
R1 |
R2 |
R1 |
R2 |
|
|
||
(284) |
|
(285) |
|
100 |
|
: |
00 |
94 |
|
: |
06 |
100 |
|
: |
00 |
70 |
|
: |
30 |
80 |
|
: |
20 |
SCHEME 61
