
Principles and Applications of Asymmetric Synthesis
.pdf

484 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
The enantioselective addition of a nucleophile to a carbonyl group is one of the most versatile methods for C±C bond formation, and this reaction is discussed in Chapter 2. Tri¯uoromethylation of aldehyde or achiral ketone via addition of ¯uorinated reagents is another means of access to ¯uorinated compounds. Tri¯uoromethyl trimethylsilane [(CH3)3SiCF3] has been used by Prakash et al.87 as an e½cient reagent for the tri¯uoromethylation of carbonyl compounds. Reaction of aldehydes or ketones with tri¯uoromethyltrimethylsilane can be facilitated by tetrabutyl ammonium ¯uoride (TBAF). In 1994, Iseki et al.88 found that chiral quaternary ammonium ¯uoride 117a or 117b facilitated the above reaction in an asymmetric manner (Scheme 8±42).
Scheme 8±42. Asymmetric tri¯uoromethylation of carbonyl compounds by chiral quaternary ammonium ¯uorides.
8.3NEW CONCEPTS IN ASYMMETRIC REACTION
8.3.1Ti Catalysts from Self-Assembly Components
As mentioned in Chapter 1, ligand-accelerated catalysis occurs when a more e¨ective chiral catalyst is obtained by replacing an achiral ligand with a chiral one. Mikami et al.89 reported a di¨erent phenomenon in which a more active catalyst was formed by combining an achiral pre-catalyst with several chiral ligands. They found that the most active and enantioselective chiral catalyst was formed in preference to other possible ligand combinations (Scheme 8±43).

486 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
product with 50% yield and 91% ee. In a similar reaction, when a catalyst derived from Ti(OPri)4, (R)-BINOL, and DIPT is used, the product is obtained in 15% yield with 40% ee. In the reaction catalyzed by a combination of Ti(OPri)4 with only DIPT, very poor yield is observed without enantioselectivity. A combination of (R)-5,50-dichloro-2,20-dihydorxy-1,10-diphenyl and (R)-BINOL with Ti(OPri)4 has been shown to be the best catalyst system for promoting highly selective and active carbonyl-ene reactions. The corresponding product can be obtained with 97% ee and 66% yield.
8.3.2Desymmetrization
Desymmetrization, which refers to a process of e½ciently desymmetrizing mesomolecules or achiral molecules to produce chiral ones, is a versatile method for preparing chiral nonracemic molecules.90 Desymmetrization of mesocompounds generally leads to the formation of a C±C or a C±X (X is a hetero atom) bond. The reaction normally uses a functional group residing on the symmetric element (in most cases the C2 axis or a plane) to di¨erentiate two (or more) symmetrically equivalent functionalities elsewhere within the substrate molecule. This work was ®rst reported by Hoye et al.91 and Mislow and Siegel92 in 1984.
Hoye demonstrated that the carbonyl group that lies on the C2-symmetric axis in keto diacid 119 can be reduced to alcohol 120. When followed by acidcatalyzed lactonization of the hydroxyl group with either a C-1 or C-9 carboxyl group, monolactone 121 or ent-121 can be produced, thus realizing the desymmetrization of substrate 119 (Scheme 8±45).
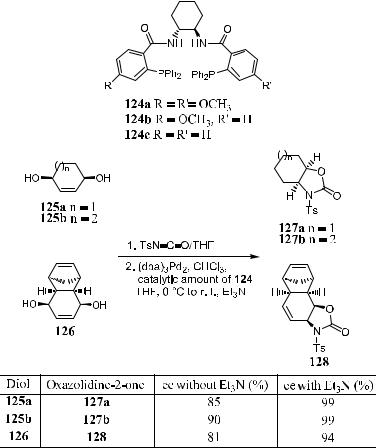
In investigating catalytic desymmetrization, Trost et al.93 demonstrated that palladium-catalyzed desymmetrization of meso-1,4-diol diesters (122a±d ) gave monosubstituted products in high ee. In the course of desymmetrization, the cleavage of the leaving group is involved in the enantiodiscriminating step (Scheme 8±46).
Desymmetrization of compound 125 can be realized in the presence of a Pd complex containing 124. Initial work showed94 that oxazolidine-2-one (R ˆ NTs) was obtained from bis-carbamates with a relatively low level of enantiomeric excess. In the presence of 1 equivalent of triethylamine, both ee and yield were enhanced dramatically (Scheme 8±47).
Miyafuji and Katsuki95 reported the desymmetrization of mesotetrahydrofuran derivatives via highly enantioselective C±H oxidation using Mn±salen catalysts. The optically active product lactols (up to 90% ee) are useful chiral building blocks for organic synthesis (Scheme 8±48).
8.3.3Cooperative Asymmetric Catalysis
Cooperative catalysis between multiple metal centers is considered to be common in enzymatic systems,96 and using this idea for designing catalytic systems

8.3 NEW CONCEPTS IN ASYMMETRIC REACTION |
487 |
|||
|
|
|
|
|
|
|
|
|
|
Scheme 8±45
Scheme 8±46
has become an interesting development in asymmetric synthesis. In most of our previous discussions, the reaction systems contain one catalysis center by which substrate and reagent are oriented and activated and the asymmetric induction is ®nally realized. Recently, there have been some interesting catalysis systems that contain two kinds of catalytic center within one catalyst. These two catalytic centers are normally two metal centers functioning harmoniously, with one
488 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
Scheme 8±47. Reprinted with permission by Am. Chem. Soc., Ref. 94.
center activating the substrate or the reagent and the other one directing the attack. These bimetallic catalytic systems may provide high chemoselectivity and/or stereoselectivity.
Reactions involving bimetallic catalysts, either homo-dinuclear or heterobimetallic complexes, and chemzymes were highlighted by Steinhagen and Helmchen96c in 1996. Some examples are discussed in Chapter 2. Among these examples, Shibasaki's reports have been of particular signi®cance.97 Shibasaki's catalyst is illustrated as 130, which consists of one central metal M1 (La‡3, Ba‡2, or Al‡3), three other metal ions (M2)‡ [(M2)‡ can be Li‡, Na‡, or K‡], and three bidentated ligands, such as (R)- or (S)-BINOL. The catalyst exhibits both Lewis acidic properties because of the existence of central metal and the Lewis basic properties because of the presence of the outer metal ions.
The multifunctional catalysts constitute a new class of widely applicable and

|
8.3 NEW CONCEPTS IN ASYMMETRIC REACTION |
489 |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 8±48
versatile chiral catalysts and can be used for many asymmetric reactions. It has been reported that asymmetric nitroaldol condensation (the Henry reaction, Chapter 3), aldol reaction (Chapter 3), hydrophosphonylation of imides (Chapter 2), Sharpless epoxidation (Chapter 4), desymmetrization of meso-epoxide (Chapter 4), BINAL±H reduction (Chapter 6), conjugate addition,97f,g tandem Michael-aldol addition,97j and 1,4-addition of Grignard reagent to enones98 can all be catalyzed by this type of catalyst.
As shown in Scheme 8±49, this multifunctional catalyst can be applied in direct aldol reactions between an aldehyde R1CHO and a ketone R2COCH3.99


8.3 NEW CONCEPTS IN ASYMMETRIC REACTION |
491 |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 8±6. Transition state for 131 catalyzed asymmetric cyanosilylation.
conditions, giving the corresponding product with high yield and enantioselectivity. The reaction transition state is shown in Figure 8±6. In the course of the reaction, the phosphine oxide group activates cyanosilylation reagent TMSCN, and the central Al atom activates the aldehyde, thus facilitating the reaction. As proposed by Shibasaki, there might be two competing reaction pathways in the case of more reactive aldehydes. The desired pathway, which gives enantioselective product, may involve the dual interaction between the aldehyde and the Lewis acid and also between the phosphine oxide and TMSCN, whereas the undesired pathway involves the interaction of the Lewis acid and aldehyde only. Shibasaki further proposed that the rate of the two pathways could be made to di¨er signi®cantly from each other if the Lewis acidity of the catalyst were decreased. The purpose of the phosphite additive is to reduce the Lewis acidity of the central Al atom via phosphite coordination, thus increasing the enantioselectivity of the reaction. In Scheme 8±50, the cyanosilylation of a series of aldehydes occurs at ÿ40 C, and in most cases both the yield and the ee are over 90%.
Another example of this cooperative catalysis has been presented by Konsler et al.101 in the course of their asymmetric ring-opening (ARO) study. They found that the ARO of meso-epoxides with TMS-N3, catalyzed by Cr±salen compound 132, showed a second-order kinetic dependence on the catalyst.102 They then proposed that there might be cooperative, intramolecular bimetallic catalysis taking place, with one metal activating the substrate meso-epoxide and

492 ENZYMATIC REACTIONS AND MISCELLANEOUS ASYMMETRIC SYNTHESES
another metal activating the nucleophile TMS-N3. Based on this assumption, new catalyst 133, linking two Cr±salen moieties together, was synthesized and tested for its ability to catalyze ARO reactions.
Their study showed that when compounds 133 catalyze asymmetric ring opening with cyclopentene oxide the reaction rate was signi®cantly enhanced, especially when the reaction was catalyzed by 133c (n ˆ 5). Even if catalyst 133c was used at a very low concentration, the reaction still proceeded very rapidly, providing the ring-opening product with comparable ee to that catalyzed by the monomeric analogs at much higher concentration. In designing the catalyst, it is essential to use a ¯exible tether of proper length so as to get the optimal transition state entropy and enthalpy.
8.3.4Stereochemical Nonlinear Effects in Asymmetric Reaction
Nonlinear e¨ects in asymmetric reaction (NLEs) refer to the nonlinear relationships between the ee value of the chiral auxiliary or ligand and the ee value in the product.103
The application of a chiral auxiliary or catalyst, in either stoichiometric or catalytic fashion, has been a common practice in asymmetric synthesis, and most of such auxiliaries are available in homochiral form. Some processes of enantiodi¨erentiation arise from diastereomeric interactions in racemic mixtures and thus cause enhanced enantioselectivity in the reaction. In other words, there can be a nonlinear relationship between the optical purity of the chiral auxiliary and the enantiomeric excess of the product. One may expect that a chiral ligand, not necessarily in enantiomerically pure form, can lead to high levels of asymmetric induction via enantiodiscrimination. In such cases, a nonlinear relationship (NLE) between the ee of the product and the ee of the chiral ligand may be observed.
In 1986, Puchot et al.104 studied the nonlinear correlation between the enantiomeric excess of a chiral auxiliary and the optical yield in an asymmetric synthesis, either stoichiometric or catalytic. Negative NLEs [(ÿ)-NLEs] were observed in the asymmetric oxidation of sul®de and in [S]-proline±mediated asymmetric Robinson annulation reactions, while a positive NLE [(‡)-NLEs]
