
Radiation Physics for Medical Physiscists - E.B. Podgorsak
.pdf
|
7.11 Example 2: Interaction of 8 MeV Photons with Copper |
259 |
|||||||||||||||
where |
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
aτ |
0.004 |
= 1.3 × 10−3, |
|
||||||||||
wτ = |
|
|
|
|
|
= |
|
|
|
|
(7.133) |
||||||
aµ |
3.051 |
||||||||||||||||
wσ = |
aσc |
= |
|
1.737 |
= 0.57, |
(7.134) |
|||||||||||
aµ |
3.051 |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
wκ = |
aκ |
|
= |
1.310 |
|
= 0.43, |
(7.135) |
||||||||||
aµ |
3.051 |
||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||
|
|
trτ = hν − PKωK |
|
K = 8 MeV − 0.5 × 0.85 × 7.7 × 10−3 MeV |
|||||||||||||
|
E |
hν |
|||||||||||||||
|
|
≈ 8 MeV, |
|
|
(7.136) |
||||||||||||
(see Figs. 7.24 and 7.25 for values of PK, ωK, and hνK)
|
|
trσ |
= 0.67 × 8 MeV ≈ 5.36 MeV, |
(7.137) |
|
E |
|||
(see “The Compton Graph” in Fig. 7.8) |
|
|||
|
|
trκ |
= hν − 2mec2 = 8 MeV − 1.02 MeV ≈ 7 MeV. |
(7.138) |
|
E |
|||
Inserting into (7.121) the weights wi and the average energy transfers Eitr for the individual e ects, we now calculate the average energy transferred from 8 MeV photons to charged particles in copper
Etr = 1.3 × 10−3 × 8 MeV + 0.57 × 5.36 MeV + 0.43 × 7 MeV
= 6.07 MeV . |
(7.139) |
5.The mass energy transfer coe cient µtr/ρ is determined from the following:
|
|
|
|
|
|
cm2 6.07 |
|
|
|||||
µtr |
|
µ Etr |
|
|
|
||||||||
|
= |
|
|
|
|
|
= 0.0289 |
|
|
|
= 0.0219 cm2 |
/g . |
(7.140) |
ρ |
|
ρ hν |
|
|
|||||||||
|
|
|
g 8 |
|
|
||||||||
6.The radiative fraction g¯ represents an average radiative yield B(EKo) given in (5.49) for the spectrum of charged particles released by 8 MeV photons in the copper absorber. This charged particle spectrum is composed of recoil Compton electrons (average energy of 5.36 MeV) as well as electrons and positrons from the pair production (average energy of 0.5×7 MeV, i.e., 3.5 MeV).
The Etr exceeds Eo, the average of the initial energies acquired by charged particles that are set in motion in the absorber, because in pair production two charged particles with a combined energy of 7 MeV are set in motion and the initial average energy for each of the two charged particles is 3.5 MeV.
260 |
7 Interactions of Photons with Matter |
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
|
The average initial energy |
|
o of all charged particles released in copper |
|||||||||||||||||||||||||||||||||||
|
E |
|||||||||||||||||||||||||||||||||||||
|
by 8 MeV photons is given as |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
o = |
|
|
tr |
|
aσ + aκ |
= 6.07 MeV |
1.737 + 1.310 |
|
|
||||||||||||||||||||||||
|
|
E |
E |
|
||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.737 + 2 × 1.310 |
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
aσ + 2aκ |
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
= 4.25 MeV. |
|
|
|
|
|
|
|
|
|
(7.141) |
|||||||||||||||||||||||
|
The spectrum of charged particles released by 8 MeV photons in the |
|||||||||||||||||||||||||||||||||||||
|
copper absorber can only be determined reliably by Monte Carlo calcu- |
|||||||||||||||||||||||||||||||||||||
|
lations. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
In the first approximation, however, we assume that all charged particles |
|||||||||||||||||||||||||||||||||||||
|
are produced with monoenergetic initial energies |
|
o. Then the radiative |
|||||||||||||||||||||||||||||||||||
|
E |
|||||||||||||||||||||||||||||||||||||
|
yield B(EKo), given in Fig. 5.7, can be equated with the radiative fraction |
|||||||||||||||||||||||||||||||||||||
|
g¯ to get g¯ ≈ 0.1. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
The average energy |
E |
rad radiated by charged particles as bremsstrahlung |
|||||||||||||||||||||||||||||||||||
|
is given by (5.50) as |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
rad = B(EKo) |
|
|
tr = 0.1 × 6.07 MeV = 0.61 MeV. |
(7.142) |
||||||||||||||||||||||||||||||
|
|
E |
E |
|||||||||||||||||||||||||||||||||||
7. The average energy |
|
|
ab absorbed in the copper absorber is |
|
||||||||||||||||||||||||||||||||||
E |
|
|||||||||||||||||||||||||||||||||||||
|
|
|
|
ab = |
|
|
tr − |
|
|
rad = 6.07 MeV − 0.61 MeV |
|
|||||||||||||||||||||||||||
|
|
E |
E |
E |
|
|||||||||||||||||||||||||||||||||
|
|
|
|
|
|
= 5.46 MeV. |
|
|
|
|
|
|
|
|
|
(7.143) |
||||||||||||||||||||||
8. The mass energy absorption coe cient µab/ρ is |
|
|||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cm2 5.46 |
|
||||||||||||||||
|
|
|
µab |
|
|
|
|
µ Eab |
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
= |
|
|
|
|
|
|
|
|
|
|
|
|
= 0.0289 |
|
|
|
|
|
= 0.0197 cm2/g |
(7.144) |
||||||||||||
|
|
|
ρ |
|
ρ hν |
g |
8 |
|
||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
The mass energy absorption coe cient µab/ρ may also be calculated from |
|||||||||||||||||||||||||||||||||||||
|
the mass energy transfer coe cient µtr/ρ and the radiative fraction g¯ as |
|||||||||||||||||||||||||||||||||||||
|
follows: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
µab |
|
|
|
µtr |
|
|
|
|
|
|
|
|
|
cm2 |
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
= |
|
|
|
(1 − g¯) = 0.0219 |
|
|
|
(1 − 0.1) |
|
|||||||||||||||||||||||||
|
|
|
ρ |
|
|
ρ |
|
|
g |
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
= 0.0197 cm2/g. |
|
|
|
|
|
|
|
|
|
(7.145) |
||||||||||||||||||||||
In summary, as shown schematicall in Fig. 7.37, we determined for 8 MeV photons interacting with a copper absorber that on the average:
(a)6.07 MeV will be transferred to charged particles; 1.93 MeV will be scattered.
(b)5.46 MeV will be absorbed in copper; 0.61 MeV will be radiated in the form of bremmstrahlung.
(c)The atomic cross-section aµ, the mass attenuation coe cient µm, and the linear attenuation coe cient µ for 8 MeV photons in copper are: 3.051 b/atom; 0.0289 cm2/g; and 0.259 cm−1, respectively.

7.11 Example 2: Interaction of 8 MeV Photons with Copper |
261 |
(d)The mass energy transfer coe cient µtr/ρ and mass energy absorption coe cient µab/ρ are: 0.0219 cm2/g and 0.0197 cm2/g, respectively.
(e)The radiative fraction g¯ for 8 MeV photons in copper is 0.1.
Fig. 7.37. Schematic diagram for general photon interactions with an atom. In this example an 8 MeV photon hν interacts with a copper atom. An individual 8 MeV photon, as it encounters a copper atom at point A, may interact with the atom through photoelectric e ect, Rayleigh scattering, Compton e ect or pair production, or it may not interact at all. However, for a large number of 8 MeV photons striking copper, we may state that on the average:
•6.07 MeV will be transferred at point A to charged particles (mainly to fast energetic electrons, but possibly also to positrons if the interaction is pair production);
•1.93 MeV will be scattered through Rayleigh and Compton scattering (hν )
Of the 6.07 MeV transferred to charged particles:
•5.46 MeV will be absorbed in copper over the fast charged particle tracks,
•0.61 MeV will be emitted in the form of bremsstrahlung photons (hν )
•The average energies transferred to charged particles in a photoelectric process, Rayleigh scattering, Compton scattering, and pair production are 8 MeV, 0, 5.36 MeV, and 7 MeV.
Teletherapy with a Cobalt-60 Source
The two figures on the next page depict a cobalt-60 teletherapy machine: actual modern machine on the left and a schematic diagram on the right. The machine is manufactured as “a megavoltage external beam therapy system using cobalt technology” by MDS Nordion in Ottawa, Canada. The schematic diagram of a cobalt machine was presented on a Canadian stamp issued in 1988 by the Canada Post Corporation in honor of Harold E. Johns (1915–1997), a Canadian medical physicist and the inventor of the cobalt-60 teletherapy machine.
The cobalt machine, shown schematically on the stamp, was developed in Canada in the 1950s for use in cancer therapy. It was the first truly practical and widely available megavoltage cancer therapy machine and incorporates a radioactive cobalt-60 source that is characterized with features suitable for external beam radiotherapy, such as high gamma ray energy, relatively long half-life, and high specific activity.
The cobalt-60 source is produced in a nuclear reactor by irradiating the stable cobalt-59 nuclide with thermal neutrons. The cobalt-60 source decays with a half-life of 5.26 years to nickel-60 with emission of beta particles (electrons) and two gamma rays (1.17 MeV and 1.33 MeV) per each disintegration, as shown schematically on the stamp.
Most modern cobalt machines are arranged on a gantry so that the source may rotate about a horizontal axis referred to as the machine isocenter axis. The sourceaxis distance typically is either 80 cm or 100 cm depending on the machine design. The isocentric source mounting allows the use of the isocentric treatment technique in which the radiation beam is directed toward the patient from various directions thereby concentrating the radiation dose in the target and spreading the dose to healthy tissues over a larger volume.
During the past two decades the linear accelerator (linac) eclipsed the cobalt unit and became the most widely used radiation source in modern radiotherapy. Compared to cobalt units linear accelerators o er higher beam energies that result in better skin sparing e ect and more e ective penetration into tissue; higher output dose rates that result in shorter treatment times; electron beams in addition to photon beams for treatment of superficial lesions; and a possibility for beam intensity modulation that provides optimal dose distributions in the target volume.
Despite the technological and practical advantages of linear accelerators over cobalt-60 machines, the latter still occupy an important place in the radiotherapy armamentarium, mainly because of considerably lower capital, installation and maintenance costs in comparison with linear accelerators. Moreover, the design of modern cobalt machines o ers many of the features that until lately were in the domain of linear accelerators, such as large source-axis distance, high output, dynamic wedges, independent jaws, and a multileaf collimator. In the developing world, the cobalt-60 machines, owing to their relatively low costs as well as simpler design, maintenance, and operation, are likely to play an important role in cancer therapy for the foreseeable future.
Left photo: Courtesy of MDS Nordion, Ottawa, Canada. Reproduced with permission.
Right photo: c Canada Post Corporation. Reproduced with Permission c Soci´et´ canadienne des postes. Reproduit avec permission.

8 Radioactivity
In this chapter we discuss various aspects of radioactivity of importance in medical physics. Natural radioactivity was discovered by Henri Becquerel in 1896, artificial radioactivity by Fr´ed´eric Joliot and Ir`ene Joliot-Curie in 1934. Artificial production of radionuclides (nucleosynthesis) plays an important role in the treatment of cancer with teletherapy and brachytherapy as well as in nuclear medicine imaging. Radioactivity is a process by which an unstable parent nucleus decays into a more stable daughter nucleus that may or may not be stable. The unstable daughter nucleus will decay further until a stable nuclear configuration is reached. The radioactive decay is governed by the formalism based on the definition of activity and the radioactive decay constant.
We first discuss the decay of an unstable parent nucleus into a stable daughter nucleus, and then develop a formalism that deals with radioactive series decay and with the activation of radionuclides. A brief discussion of the origin of radioactive nuclides is then given, followed first by a discussion of general aspects of the radioactive decay and then by a detailed description of the various radioactive decay modes available to radioactive nuclides in their quest to attain a more stable configuration. Special emphasis is placed on aspects of radioactive decay of importance to medical physics.
264 8 Radioactivity
8.1 Introduction
Radioactivity, discovered in 1986 by Henri Becquerel, is a process by which an unstable parent nucleus transforms spontaneously into one or several daughter nuclei that are more stable than the parent nucleus by having larger binding energies per nucleon than does the parent nucleus. The daughter nucleus may also be unstable and will decay further through a chain of radioactive decays until a stable nuclear configuration is reached. Radioactive decay is usually accompanied by emission of energetic particles that may be used in science, industry, agriculture, and medicine.
•Nuclear decay, also called nuclear disintegration, nuclear transformation or radioactive decay, is a statistical phenomenon.
•The exponential laws that govern nuclear decay and growth of radioactive substances were first formulated by Ernest Rutherford and Frederick Soddy in 1902 and then refined by Harry Bateman in 1910.
•A radioactive substance containing atoms of same structure is often referred to as radioactive nuclide. Radioactive atoms, like any other atomic structure, are characterized by the atomic number Z and atomic mass number A.
•Radioactive decay involves a transition from the quantum state of the original nuclide (parent) to a quantum state of the product nuclide (daughter). The energy di erence between the two quantum levels involved in a radioactive transition is referred to as the decay energy. The decay energy is emitted either in the form of electromagnetic radiation (usually gamma rays) or in the form of kinetic energy of the reaction products.
•The mode of radioactive decay depends upon the particular nuclide involved.
•All radioactive decay processes are governed by the same general formalism that is based on the definition of the activity A(t) and on a character-
istic parameter for each radioactive decay process: the total radioactive decay constant λ with dimensions of reciprocal time, usually in s−1.
•The decay constant λ is independent of the age of the radioactive atom and is essentially independent of physical conditions such as temperature, pressure, and chemical state of the atom’s environment. Careful measurements have shown that λ can actually depend slightly on the physical environment. For example, at extreme pressure or at extremely low tem-
perature the technetium-99m radio-nuclide shows a fractional change in λ of the order of 10−4 in comparison to the value at room temperature (293 K) and standard pressure (101.3 kPa).
•The total radioactive decay constant λ multiplied by a time interval that is much smaller than 1/λ represents the probability that any particular atom of a radioactive substance containing a large number N (t) of identical radioactive atoms will decay (disintegrate) in that time interval. An assumption is made that λ is independent of the physical environment of a given atom.

8.2 Decay of Radioactive Parent into a Stable Daughter |
265 |
•The activity A(t) of a radioactive substance containing a large number N (t) of identical radioactive atoms represents the total number of decays (disintegrations) per unit time and is defined as a product between N (t) and λ, i.e.,
A(t) = λN (t) . |
(8.1) |
•The SI unit of activity is the becquerel (Bq) given as 1 Bq = 1 s−1. The becquerel and hertz both correspond to s−1, but hertz expresses frequency of periodic motion, while becquerel expresses activity.
•The old unit of activity, the curie (Ci), was initially defined as the activity of 1 g of radium-226 and given as 1 Ci = 3.7×1010 s−1. The activity of 1 g of radium-226 was subsequently measured to be 3.665×1010 s−1; however,
the definition of the curie was kept at 3.7 × 1010 s−1. The current value of the activity of 1 g of radium-226 is thus 0.988 Ci or 3.665 × 1010 Bq.
•The becquerel (Bq) and the curie (Ci) are related as follows: 1 Bq = 2.703 × 10−11 Ci or 1 Ci = 3.7 × 1010 Bq.
•The specific activity a is defined as activity A per unit mass M , i.e.,
a = |
A |
= |
λN |
= |
λNA |
, |
(8.2) |
|
|
M |
A |
||||||
|
M |
|
|
|
|
|
||
where NA is the Avogadro’s number (6.022 × 1023 atom/g-atom).
The specific activity a of a radioactive species depends on the decay constant λ and on the atomic mass number A of the radioactive atom. The units of specific activity are Bq/kg (SI unit) and Ci/g (old unit).
8.2 Decay of Radioactive Parent into a Stable Daughter
The simplest form of radioactive decay is characterized by a radioactive parent nucleus P decaying with decay constant λP into a stable daughter nucleus D, i.e.,
λP |
(8.3) |
P −→ D . |
The rate of depletion of the number of radioactive parent nuclei NP(t) is equal to the activity AP(t) at time t, i.e.,
dNP(t) |
= −AP(t) = −λPNP(t) . |
(8.4) |
dt |
The fundamental di erential equation of (8.4) for NP(t) can be rewritten in general integral form to get
NP(t) |
NP |
= − 0 |
t |
(8.5) |
|
λPdt , |
|||
|
dNP(t) |
|
|
|
NP(0)
where NP(0) is the number of radioactive nuclei at time t = 0.
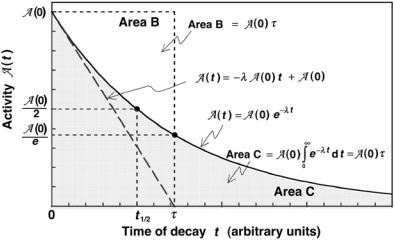
8.2 Decay of Radioactive Parent into a Stable Daughter |
267 |
Fig. 8.1. Activity A(t) plotted against time t for a simple decay of a radioactive parent into a stable daughter. The activity follows the relationship given in (8.8) and (8.15). The concepts of half-life t1/2 and mean-life τ are also illustrated. The area under the exponential decay curve from 0 to ∞ is equal to A(0)τ where A(0) is the initial activity of the parent nuclei. The slope of the tangent to the decay curve at t = 0 is equal to −λAP(0) and this tangent crosses the abscissa axis at t = τ
The decay constant λP and mean life τP are related through the following expression:
τP = |
1 |
. |
(8.13) |
|
|||
|
λP |
|
|
The mean life τP can also be defined as the time required for the number of radioactive atoms or their activity to fall to 1/e = 0.368 of its initial value NP(0) or initial activity AP(0), respectively.
The mean life τP and half-life (t1/2)P are related as follows:
τP = |
1 |
= |
(t1/2)P |
= 1.44(t1/2)P . |
(8.14) |
|
ln 2 |
||||
|
λP |
|
|
||
A typical example of a radioactive decay for initial condition AP(t = 0) = AP(0) is shown in Fig. 8.1 with a plot of parent activity AP(t) against time t, i.e.,
AP(t) = AP(0)e−λPt . |
(8.15) |
The following properties of the radioactive decay curve are notable:
1.The area under the activity AP(t) vs. time t curve for 0 ≤ t ≤ ∞ is given as

268 8 Radioactivity
∞ |
∞ |
AP(t)dt = AP(0) |
−λPtdt |
|
|
|
|
|||
0 |
0 |
|
|
|
|
|
|
|
= |
AP(0) |
= |
AP |
(0)τ |
|
= N |
(0) , |
(8.16) |
|
λP |
|
P |
P |
|
|
||
and the result equals the initial number of radioactive nuclei at time t = 0.
2.The total number of radioactive nuclei present at any time t > 0 is simply the activity AP(t) multiplied by the mean life τP.
3.The concept of half-life (t1/2)P is shown in Fig. 8.1 as the time in which the activity AP(t) drops from AP(0) to 0.5 AP(0).
4.The concept of mean life τP is shown in Fig. 8.1 as the time in which the activity AP(t) drops from AP(0) to 0.368AP(0).
5.Area AP(0)τP is shown in Fig. 8.1 by a rectangle with sides AP(0) and τP. If the initial activity AP(0) could remain constant for mean life τ , all atoms would have been transformed and at time t = τP the activity would drop to zero.
6.In general, the slope of the tangent to the decay curve at time t is given as
dAP(t) |
= |
λ |
PAP |
(0)e−λPt , |
(8.17) |
dt |
|
− |
|
|
while the initial slope at t = 0 is equal to −λPAP(0).
7.The linear function, with the slope equal to −λPAP(0) and the ordinate intercept at time t = 0 equal to AP(0), is
AP(t) = −λPAP(0)t + AP(0) , |
(8.18) |
and represents the tangent to the decay curve at t = 0. It serves as a good approximation for the activity AP(t) vs. t relationship when t τP, i.e.,
AP(t) ≈ AP(0){1 − λPt} = AP(0) {1 − t/τP} |
(8.19) |
and results in AP(t) = 0 at t = τP, in contrast to (8.15) that predicts AP(t) = 0 only at t → ∞.
8.3 Radioactive Series Decay
8.3.1 Parent → Daughter → Granddaughter Relationships
Equations (8.7) and (8.15) describe the simple radioactive decay from an unstable parent P to a stable daughter D. A more complicated radioactive decay occurs when a radioactive parent P decays with a decay constant λP into a daughter D that in turn is radioactive and decays with a decay constant λD into a stable granddaughter G, i.e.,
λP |
λD |
(8.20) |
P −→ D −→ G . |
||
