
English-Повн термін-біохімія-03-Р-ни---2013
.pdf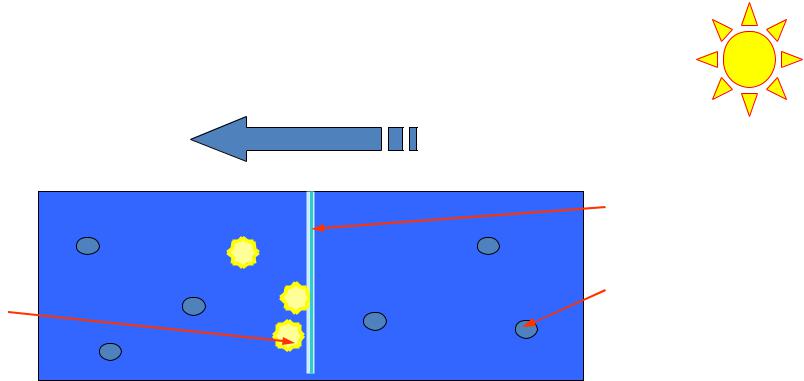
Osmosis is one way diffusion of molecules of solvent through a semipermeable membrane from solution with lower concentration into another one with higher concentration.
1
2
3
1 – a semipermeable membrane; 2 – molecules of solvent; 3 – particles of solute
Osmotic pressure is caused by lowering chemical potential of solvent in presence of solute.
21
 h
h
1  3
3

 2
2
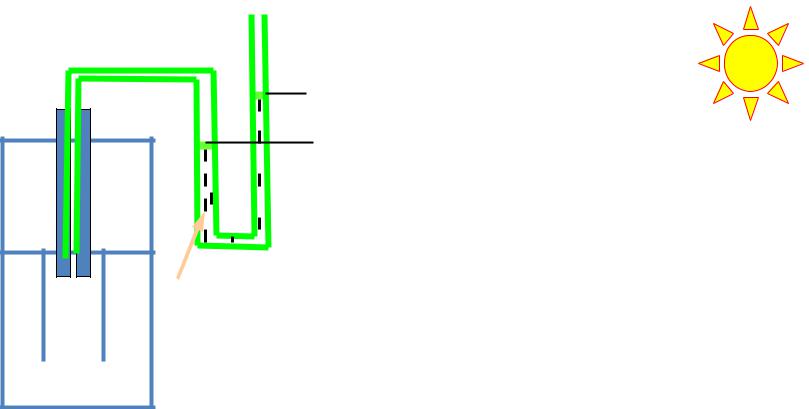
Scheme of osmometer :
1- manometer; 2- container with solution, 3- container with solvents
Extra hydrostatic pressure occurs as a result of diffusion of solvent from exterior container into container with solution. Since the level of liquid inside of tube rises to some height h in equilibrium, that forms extra hydrostatic pressure р
p = hρg,
where h – excessive height of column of liquid ; ρ – liquid density; g – acceleration of Earth gravity power.
22

J. van’t-Hoff (Я.Вант-Гофф) (1887) proposed to use gas laws for calculation of osmotic pressure :
Osmotic pressure of solution equals to the pressure of solute in a case of its gaseous state when it takes the same volume as the
solution.
The equation of gas state by Mendeleyev and Clapeyron:
pV= νRT=(mRT)/M
ideal gas constant; universal molar gas constant; R
The constant that appears in the ideal gas equation. It is equal to 8.314 34 J/K/mol.
23

The equation of osmotic pressure of solution by van’t-Hoff
π = (mRT)/(MV) = CMRT,
π– osmotic pressure of solution, кПа; CM- molar
concentration of solution, mol/L; R- universal gas constant {8,314 J/(mol К)}; Т – absolute temperature, К.
Vant-Hoff Law: Osmotic pressure of solution is directly proportional to its molar concentration and absolute temperature.
Value of osmotic pressure of solution depends on osmotic concentration (osmomolality; Cоsм)
π= (mRT)/(MV) = CоsмRT
24

Biological fluids are water solutions of many mineral and organic substances (р=constant)
The blood plasma, р = 770 – 821 кПа (7,6 – 8,1 аtm)
The blood р is caused by ions Na+ and Cl- (by 60 %), proteins – less.
Pressure formed by high molecular biologically active substances is called an oncotic pressure.
0,5 % of total osmotic pressure (3,04 –4,05 kPа or 0,03 – 0,04 atm);
by 80% is caused by albumins;
since р of lymph ~ 1,33 kPа , р of blood ~ 4 kPа, water enter from lymph into blood on account of difference between these pressures.
25

If osmotic pressure of the first solution is higher than the second one, the first solution is hyper-tonic, and in the opposite case – hypo-tonic.
Solutions with the same osmotic pressure are determined as isotonic
Isotonic solution use for infusion
In clinic practice: isotonic solutions have osmotic pressure which is equal to р of the blood plasma = 7,7 – 8,1 atm
or 0,85-0,9 % of sodium chloride, or 4,5 –5 % of glucose solution
Physiological solutions (according their composition) are similar to sea-water
26
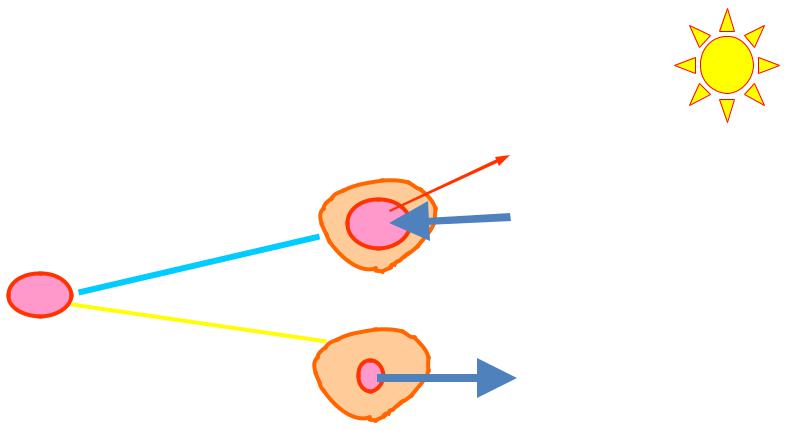
Hemolytic and plasmolytic erythrocytes
Destruction of coat of erythrocytes under the hypotonic solutions inoculation into the blood plasma (accompanied with exit of hemoglobin) is determined as hemolysis
(“лакова кров” – “polished blood”) |
Hb |
|
|
|
|
|
|
Н2О |
|
Hypotonic solution |
|
eryth- |
hemolysis |
|
|
|
|
rocyte |
|
|
|
Hypertonic solution |
|
|
|
Н2О |
|
plasmolysis |
|
Phenomenon of erythrocyte crumple after inoculation of hypertonic solutions into the blood plasma is called plasmolysis.
27

Child receiving an intravenous infusion
Saline solution for intravenous infusion
Use of hypertonic solutions
Not to ruin the osmotic equilibrium of blood hypertonic solutions of glucose (with mass fraction 20 %, 40%) inoculate into the vessels very slow (найчастіше крапельним шляхом).
Normal saline (NS) is the commonly-used term for a solution of 0.91% w/v of NaCl, about 300 mOsm/L.
Hypertonic solution sodium chloride (5 – 10 %) use in surgery for cleaning up the festered wounds (для очищення гнійних ран).
Hypertonic solutions of bitter salt (гіркої солі) MgSO4.7H2O or (глауберової солі) Na2SO4.10H2O use as purge thing (застосовують як послаблюючі засоби).
28
If you find mistakes, let me know, Sorry for inconvenience – file is under construction – L.G. K.
29
