
3 курс / Фармакология / Essential_Psychopharmacology_2nd_edition
.pdfCognitive Enhancers |
479 |
Jakob disease can cause dementia from a "slow" viral infection of the brain. Depression can cause a false dementia or pseudodementia, which can be reversed by antidepressants in many cases. In Huntington's disease, Parkinson's disease, and many other neurological disorders, dementia, such as Lewy body dementia, can be associated with various neurological signs and symptoms. These latter dementias are part of a neurodegenerative disorder that destroys various neurons in the brain, including those areas responsible for memory and cognition. Patients with acquired immunodeficiency syndrome (AIDS), often have dementia resulting from a human immunodeficiency virus (HIV) infection of the brain. Frontotemporal dementia, also called Pick's disease, can involve more frontal lobe degeneration and personality changes. There has been little systematic investigation in these various dementias of cholinergic neuronal damage or of the therapeutic benefit of cholinesterase inhibitors, although numerous anecdotal reports suggest that some patients may benefit.
Cholinesterase Inhibitors as treatments for Enhancing Memory or Slowing the Pace of Memory Loss in Alzheimer's Disease
No matter how it happens, cholinergic neuronal functioning is one of the earliest neurotransmitters to change in Alzheimer's disease, and it changes dramatically in the first year of symptoms, since the synthetic enzyme for acetylcholine, choline acetyltransferase (Fig. 12 — 7), may already be decreased by 40 to 90% in the cortex and hippocampus (Fig. 12 — 13). The nucleus basalis of Meynert also shows progressive neuronal loss in Alzheimer's disease, which correlates with the progressive loss of memory function in this disease (Fig. 12 — 13). The most successful approach to boosting cholinergic functioning in Alzheimer's patients and improving their memory has been to inhibit acetylcholine destruction by inhibiting the enzyme acetyl-cholinesterase (Fig. 12 — 23). This causes the buildup of acetylcholine because it can no longer be destroyed by acetylcholinesterase.
This pharmacologic approach has already led to the approval of two drugs for treatment of memory disorders of Alzheimer's disease in the United States, with several others in the late stages of clinical testing and approval. These drugs enhance memory and are sometimes called cognitive enhancers and sometimes promnestic (as opposed to an amnestic) agents. They are specifically approved to treat Alzheimer's disease. Since these agents appear to depend on the presence of intact postsynaptic cholinergic receptors to receive the benefits of the enhanced cholinergic input, they may be most effective in the early stages of Alzheimer's disease, while postsynaptic cholinergic targets are still present. There is some evidence that cholinesterase inhibitors may even slow the course of the underlying degenerative process in some patients, and thus may have three possible pharmacological benefits mediated by diffuse stimulation of nicotinic and muscarinic cholinergic receptors. These possible benefits are (1) functional improvement of central cholinergic neurotransmission at cholinergic synapses (especially relevant in the neocortex), mediated through muscarinic and nicotinic mechanisms; (2) protection against neuronal degeneration, mediated through nicotine receptor activation; and (3) modification of amyloid precursor protein processing, mediated through Ml receptor activation.
Donepezil is currently approved worldwide as a first-line treatment for improving memory or at least slowing the rate of memory loss in Alzheimer's disease. It is a reversible, long-acting, selective piperidine inhibitor of acetylcholinesterase (AchE)

480 Essential Psychopharmacology
FIGURE 12 — 23. Cholinesterase inhibitor treatment for Alzheimer's disease. Deficiency in cho-linergic functioning, due to degeneration in cholinergic projections from the nucleus basalis of Mey-nert, may be linked to the memory disturbance of Alzheimer's disease. Levels of acetylcholine (ACh) and its synthetic enzyme choline acetyltransferase are greatly reduced in brains of Alzheimer's patients. A powerful and successful mechanism of boosting ACh in the brain is to inhibit ACh destruction by inhibiting the enzyme acetylcholinesterase (AChE). This causes the buildup of ACh, which is no longer destroyed by acetylcholinesterase. This approach has led to the only truly effective therapies for the treatment of Alzheimer's disease. Shown here is the current first-line cholinesterase treatment in clinical practice, donepezil, which inhibits acetylcholinesterase in the cholinergic neuron and its surroundings. Other similar agents are in late clinical testing. Since these agents appear to depend on the presence of intact targets for acetylcholine for maximum effectiveness, they may be most effective in the early stages of Alzheimer's disease, before these targets degenerate. However, the cholinesterase inhibitors may actually slow the degeneration itself by releasing growth factors or by interfering with amyloid deposition.
without inhibition of butyrylcholinesterase (BuChE) (Fig. 12—24). It is easy to dose, and has mostly gastrointestinal side effects, which are mostly transient.
Tacrine was the first cholinesterase inhibitor approved for the enhancement of memory associated with Alzheimer's disease in the United States. Because of its short half-life, drug interactions, and hepatic toxicity, it is currently considered second-line therapy for patients who fail to respond to donepezil. Its psychophar-

Cognitive Enhancers |
481 |
FIGURE 12 — 24. Icon for the cholinesterase inhibitor donepezil. This is the current first-line treatment for Alzheimer's disease, since it is a once daily agent without significant hepatotoxicity. It is a reversible agent, selective for acetylcholinesterase (AChE) over butyrylcholinesterase (BuChE), developed by American and Japanese companies.
FIGURE 12-25. Icon for the cholinesterase inhibitor tacrine. This was the first cholinesterase inhibitor, but since it is a hepatoxotin, it has been relegated to second-line use. Also, it must be given four times daily, is difficult to dose, and has several drug interactions. It is short-acting, reversible, and nonselective, inhibiting both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE).
macological mechanism of action is reversible inhibition of both AchE and BuChE (Fig. 12—25). It thus has a short half-life, and must be given four times a day with falloff of enzyme inhibition and sometimes of efficacy as well, between doses. Like all cholinesterase inhibitors, its therapeutic benefits on memory and its side effects are dose-related, but because of its short duration of action, it requires very careful dose titration. Tacrine frequently causes hepatic toxicity and thus requires monitoring of liver function during drug administration. There are also several potential significant pharmacokinetic drug interactions, since tacrine is an inhibitor of CYP450 1A2, and its levels are increased by other drugs such as cimetidine. Thus, tacrine not only is a second-line treatment given the current availability of donepezil, but it will likely fall to a third-line treatment once other agents now in late clinical development are approved for clinical use.
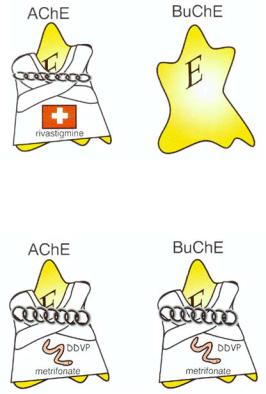
Rivastigmine is currently in the late stages of clinical development and awaiting approval for marketing in many countries. It is a carbamate, which is "pseudoirreversible" (meaning that it reverses itself over hours) and intermediate-acting, and is selective not only for AChE over BuChE, but perhaps also for AChE in the cortex and hippocampus over AChE in other areas of brain (Fig. 12 — 26). It appears to have
482 Essential Psychopharmacology
FIGURE 12-26. Icon for the cholinesterase inhibitor rivastigmine. This agent is in late development by a Swiss company. It is long-acting, pseudoirreversible, intermediate-acting, and selective for acetylcholinesterase (AChE) over butyrylcholinesterase (BuChE).
FIGURE 12-27. Icon for the cholinesterase inhibitor metrifonate. This agent is in late development for Alzheimer's disease, but is a well-known agent for schistosomiasis. It is a prodrug for 2,2-dichloro-dimethyl-phosphate (DDVP), an irreversible inhibitor of both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE).
safety and efficacy comparable with those of donepezil, although head-to-head comparisons have not been published.
Metrifonate has been used in millions of patients since the 1960s as a treatment for schistosomiasis. More recently, it has been investigated as a cognitive enhancer for Alzheimer's disease patients. Metrifonate itself is not an AChE inhibitor; rather, it is a prodrug, which is gradually converted, nonenzymatically, into another chemical, 2,2-dichlorovinyldimethyl phosphate (DDVP), which is the actual cholinesterase inhibitor. Metrifonate's psychopharmacological mechanism of action is therefore as a prodrug for an organophosphate irreversible, long-acting inhibitor of both AChE and BuChE (Fig. 12 — 27). Its onset of action is gradual, since it takes some time for DDVP to form with oral administration of metrifonate, and this can improve tolerability as the patient adjusts to cholinesterase inhibition. Some of the best studies linking dose-related AChE inhibition with clinical efficacy in improving memory in Alzheimer's disease have been made with metrifonate. Since inhibition of AChE in red blood cells by metrifonate correlates directly with the agent's inhibition of brain AChE, monitoring of red blood cell AChE has indicated that approximately 50 to 60% enzyme inhibition is sufficient to produce efficacy with acceptable tolerability. Observations of muscular weakness in some patients receiving

Cognitive Enhancers |
483 |
FIGURE 12 — 28. Icon for the cholinesterase inhibitor physostigmine. This agent is used intravenously as a short-acting cholinesterase inhibitor to reverse anticholinergic poisoning and is in testing in an oral sustained-release formulation for Alzheimer's disease.
FIGURE 12 — 29. Icon for the cholinesterase inhibitor galanthamine. This agent is naturally present in snowdrops and daffodils and may also have nicotinic agonist or cholinergic-releasing actions as well as cholinesterase actions. It is also in testing for Alzheimer's disease.
long-term treatment with metrifonate in clinical trials have caused reconsideration of dosing and safety issues prior to approval for marketing in some countries.
Physostigmine is a very short acting cholinesterase inhibitor normally used intravenously to reverse anticholinergic poisoning. It has been reformulated into an oral sustained-release preparation and successfully tested in Alzheimer's disease (Fig. 12
— 28), demonstrating efficacy in improving memory and cognition comparable to that of other cholinesterase inhibitors, but it has not yet satisfactorily resolved all safety issues (e.g., nausea and vomiting) that must be resolved for marketing to begin.
Galanthamine is a very interesting cholinesterase inhibitor found in snowdrops and daffodils. It may have a dual mechanism of action, matching cholinesterase inhibition with direct nicotinic agonist actions causing acetylcholine release (Fig. 12—29). Early testing in Alzheimer's disease is underway.
Cholinesterase inhibitors: one class of six drugs or six unique agents? Soon three to six cholinesterase inhibitors should be available worldwide for the treatment of cholinergic-related memory disturbances in Alzheimer's disease (Fig. 12 — 30). Use of these agents will likely be expanded to treat cholinergic-related behavioral disturbances in Alzheimer's disease in addition to memory disturbances, since there is evidence that behavioral problems in this disease may also respond to cholinergic

484 Essential Psychopharmacology
FIGURE 12 — 30. Alzheimer's disease pharmacy. Currently, donepezil is first-line for memory loss, and atypical antipsychotics (SDA) are first-line for positive psychotic symptoms; together they may work synergistically. Soon the cholinesterase inhibitors metrifonate and/or rivastigmine may become available. Second-line treatments are tacrine for memory and conventional antipsychotics (D2) for positive psychotic symptoms. Several other agents are in clinical and preclinical development.
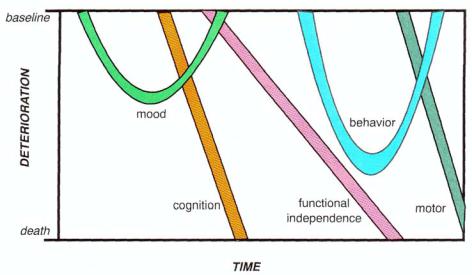
intervention (Fig. 12 — 31). Even though we have chosen to emphasize the memory impairment of Alzheimer's disease, this disorder obviously has many dimensions of functional impairments, often heralded by mood changes even before cognitive and memory declines, with consequent loss of functional independence, followed by onset of behavioral and finally motor changes (Fig. 12 — 31). The cholinesterase inhibitors may have synergistic actions with atypical antipsychotics in reducing behavioral disturbances, as discussed in Chapter 11. The cholinesterase inhibitors may also be expanded to uses outside of Alzheimer's disease, for example, to the treatment of memory disorders in other conditions, to the treatment of attention deficit disorder, and to the treatment of bipolar disorder. Thus, a thorough familiarity with these agents will be useful for the informed psychopharmacologist in the coming years, this should including knowing the relative advantages and disadvantages of each of
Cognitive Enhancers |
485 |
FIGURE 12 — 31. Time course of deterioration of multiple dimensions of symptoms in Alzheimer's disease. Changes in mood late in life, particularly if first onset in life and nonresponsive to antide-
pressants, may be a harbinger for later onset of the memory and cognitive decline of Alzheimer's disease. As cognitive decline worsens, functional impairment follows shortly thereafter. Behavioral problems then begin and become a major management issue in
this disorder. Eventually, even motor
problems develop in the last few years of life. Thus, Alzheimer's disease is certainly not just a disorder of memory, although that has been the dimension emphasized in this chapter.
the various members of this class. Given the advantages of the newer agents, there seems to be little reason to prescribe tacrine any more. Once rivastigmine and metrifonate join donepezil on the market, clinicians will want to know the relative advantages and disadvantages of each of these, however. Unfortunately, direct head- to-head comparisons in Alzheimer's disease are not yet available.
These newer drugs differ mostly in pharmacology and in type and selectivity of enzyme inhibition, which may translate more directly into differences in tolerability and drug interactions than into differences in efficacy. Changes in memory and cognition seem to be about the same for all of the cholinesterase inhibitors when tested against placebo in trials without comparators. Nevertheless, there will undoubtedly be debates on the relative advantages of reversible (short-acting) versus pseudoirreversible (intermediate-acting) versus irreversible (long-acting) enzyme inhibition, as well as on the desirability of selective versus nonselective enzyme inhibition of acetylcholinesterase versus butyrylcholinesterase and of selective versus nonselective inhibition of acetylcholinesterase in various brain regions. Currently, the manner in which these pharmacologic distinctions will translate into clinical advantages or special niche uses for one drug over another is not known and is only likely to be discovered after the compounds have been used extensively in clinical practice. However, it is theoretically possible that drugs that do not inhibit BuChE will have better tolerability, since side effects may be enhanced by the presence of increased ACh in certain tissues. On the other hand, BuChE is present in glia as well as in the plaques, tangles, and amyloid-containing blood vessels in the brains of Alzheimer patients. It is theoretically possible but unproven that inhibiting BuChE at these sites would have a useful boosting function for improving memory.
486Essential Psychopharmacology
The spectrum of potential memory enhancing benefits of the cholinesterase inhibitor class of therapeutic agents. Cholinergic enhancement compensates for the loss of ACh that occurs as cholinergic neurons degenerate. The cholinergic enhancement strategy has yielded the only successful therapy for improving memory in any cognitive disorder and is specifically approved for treating cognitive symptoms in Alzheimer's disease, but it has obvious limitations. For instance, we have already mentioned that the ideal pharmacologic situation is likely to present itself early in the illness, when postsynaptic neurons and their cholinergic receptors in the cortex are still intact, even though presynaptic cholinergic inputs from the nucleus basalis of Meynert have degenerated. However, not only is it difficult to diagnose patients at this stage of the illness, but it is particularly difficult to monitor the effects of treatment, because the available rating scales are not very sensitive in picking up subtle changes, even in a research setting. Another limitation of the cholinergic approach to enhancing memory in Alzheimer's disease is that as the illness advances, the postsynaptic neurons in the neocortex degenerate, removing the targets for acetylcholine. Furthermore, replacing acetylcholine will not improve functions mediated by the loss of those noncholinergic neurotransmitters.
Studies of the untreated course of Alzheimer's disease (Fig. 12 — 13), coupled with extensive long-term experience with two cholinesterase inhibitors in clinical practice and several more in clinical trials over the past few years, are helping to set expectations for what these agents can achieve in terms of memory enhancement. As with many psychopharmacological agents, the median response rate of a large group of patients often belies the range of responses exhibited by individuals, and since there is no way of predicting who will experience the more robust clinical responses, only empirical trial and error of individual patients can ultimately tell who will be helped the most by these agents. Nevertheless, the range of responses is well known and is summarized in Figures 12 — 32 to 12 — 34.
The best responses to cholinesterase inhibitors can be substantial improvement, large enough to be noticeable by the patient and his or her caregiver within weeks of initiation of therapy (Fig. 12 — 32). Some of these patients sustain this robust improvement for many months or have a noticeably slower than expected decline in memory (Fig. 12 — 32). The usual (median) response, however, is for the initial improvement to be statistically detectable on cognitive testing and perhaps to be noticeable by the caregiver, but often not by the patient. Such a response usually lasts about 6 months, and then cognitive functioning, as measured on cognitive testing, is back to where it was before beginning the drug (Fig. 12 — 33). This response is clearly drug-related, because if the drug is stopped, cognitive function immediately declines back to what would be expected if the patient had never been treated. Thereafter, the decline may be at about the same rate as before taking the drug (Fig. 12 — 33). Yet another response to cholinesterase inhibition can be to have no immediate improvement but a definite slowing in the expected rate of decline (Fig. 12 — 34). Of course, some patients do not respond at all, but it is distinctly unusual for a patient to worsen on cholinesterase inhibitor treatment.
As mentioned earlier, all cholinesterase inhibitors seem to have the same ability to improve cognitive function as compared with placebo in large clinical trials, and there are few anecdotes and no head-to-head trials suggesting that patients who do not respond robustly to one cholinesterase agent will respond robustly to another. Substantial improvement in 30-week studies might thus be expected in about one-

Cognitive Enhancers |
487 |
FIGURE 12 — 32. The best responses to cholinesterase inhibitor therapy in Alzheimer's disease can be substantial improvement, large enough to be noticeable to the patient and to his or her caregiver within weeks of initiation of therapy. Some of these patients sustain this robust improvement for many months or have a noticeably slower than expected decline in memory.
fourth of patients treated with a cholinesterase inhibitor (and only in 8 to 10% of those given placebo); about 56 to 60% are expected to show either no further deterioration or moderate improvement with the drug (versus 50% or fewer with placebo, this difference being statistically significant).
These findings from the natural history of untreated Alzheimer's disease and how it is modified by cholinesterase inhibitors should help prescribers set realistic expectations for treatment with AChE inhibitors. The hope is that today's improvements from cholinesterase inhibition will be synergistic when combined with agents of differing pharmacologic mechanisms of action in the future and should be considered a very useful first step in treating this devastating illness.

488 Essential Psychopharmacology
FIGURE 12 — 33. Usual (median) responders to cholinesterase inhibitor therapy in Alzheimer's disease. The usual (median) response to cholinesterase inhibitor therapy is for the initial improvement to be statistically detectable on cognitive testing and perhaps noticeable to the caregiver, but often not to the patient. Such a response usually lasts about 6 months, and then cognitive functioning as measured on cognitive testing is back to where it was before beginning the drug. This response is clearly drug-related, because if the drug is stopped, cognitive function declines back to what would be expected if the patient had never been treated. Thereafter, the decline may be at about the same rate as before taking the drug.
Other and Future Memory and Cognitive Enhancers
Innovation in the area of cognitive enhancers in general and in Alzheimer's disease in particular is one of the most active research areas in psychopharmacology. Although this is a most exciting topic, it may not be of interest to every reader, and especially not to the beginner or to the generalist. These readers may wish to skip to the end of the chapter and to the summary.
