
Indications for surgical treatment
The popularity of medical management of patients with aortic dissections has oscillated over the last two decades with parallel swings in the indications for surgery. The enthusiasm for nearly universal surgical treatment and resultant poor results which followed DeBakey's 1965 report [17] was probably due to misinterpretation of the implications of that study. It also illustrated the danger inherent in extrapolating the surgical results attained in a selected, nonrepresentative patient population to all patients. Currently, the pendulum has swung back toward a more aggressive surgical therapeutic philosophy, and a general consensus of opinion exists concerning the indications for surgery in most patients (see Chapter 7 lor mclication.s for medical thor.ip) ol aortic dissection) [10-12,22 24.28.2<J. 31,34,35]. It is critically important, however, to define precisely both the acuity and type of the dissection when discussing operative indications.
Acute Type A Dissections
Because of the unacceptably low survival rates of patients with acute type A
aortic dissections treated medically, most authorities currently favor emergency or
urgent surgery for these patients [4,9-13,22-24,31-35,40,41]. Exceptions, in my opinion, include patients who are not operative candidates in general for reasons unrelated to their cardiovascular system (e.g., neoplasm, other debilitating chronic
illnesses, etc.) and possibly, also, patients who have sustained irreversible cerebral, renal, or visceral infarction as a complication of the dissection [10,32,33]. Such preoperative complications limit the hope of meaningful rehabilitation but cannot be considered absolute contraindication as special circumstances exist. Dissection-induced paraplegia is probably not reversible and is associated with a high operative risk [10], but it does not proscribe operation, particularly in young patients. These guidelines are generally agreed upon by most experienced cardiologists and cardiac surgeons.
Chronic Type A Dissections
My personal philosophy restricts operation for patients with chronic type A aortic dissections. Patients who have survived the initial insult are not generally considered to have a high attrition rate [9,11-13]. It must be remembered, however, that fewer than 10 percent of untreated patients will survive the acute catastrophe, and few, if any, major medical centers in this country currently recommend medical therapy alone for patients with acute type A dissections. Thus, patients with chronic type A dissections are relatively uncommon today. Indications for operation include the development of symptoms clearly referable to the dissection or its complications, such as congestive heart failure secondary to aortic insufficiency, angina pectoris secondary to compromise of the coronary arteries, chest pain due to saccular enlargement of the ascending aorta, transient ischemic attacks due to cerebrovascular insufficiency, or upper extremity ischemia. Most of these patients present with chronic aortic insufficiency and congestive failure (see Chapter 4). The second indication includes documented progressive enlargement of a saccular aneurysmal component of the ascending aorta. Pressler and McNamara have recently shown convincingly that patients with thoracic aneurysms (dissections or atherosclerotic aneurysms) larger than 10 cm in diameter are at high risk [42,43]: 75 percent of patients with ascending aortic aneurysms were dead within 2 years, and aortic rupture was the cause of death in 77 percent of patients with dissections [43]. As was the case for patients with acute type A dissections, individual judgment is necessary if the patient is elderly and harbors other serious systemic illnesses or disabilities that limit life expectancy. Thus, the third indication for operative intervention is the presence of a saccular component that exceeds 10 cm in diameter, even in the absence of documented enlargement or symptoms.
On the other hand, there is little or no evidence supporting elective surgical treatment for patients with chronic, asymptomatic dissections that are not enlarging and do not exceed 10 cm in diameter. Such patients, however, should be followed carefully to detect any increase in size which might portend rupture. Computed tomographic (CT) scanning has proven to be most effective in this regard in our experience (see also Chapter 6) [36].
Acute Type B DtttcctiOfM
It is this subgroup consisting of patients with acute type B aortic dissections which generates the most controversy and debate concerning surgical versus medical therapy (see Chapter 7 for discussion supporting medical therapy for uncomplicated acute type B dissection) [9-13,20,22,23,27,30-33,44]. Autopsy studies over the years [1-3,26] (going back to Peacock in 1843) [25] have revealed that the prognosis of undiagnosed and untreated patients with type B dissections was superior to that of patients with type A dissections. Dinsmore and his colleagues at the Massachusetts General Hospital confirmed this fact in 1972 when the angio-graphic appearance was correlated with prognosis [44]. The overall survival rate was even better if the false lumen did not opacify at the time of angiography. On the other hand, when a medical regimen fails to control pain or progression of the dissection or when major complications of the dissection occur during medical treatment of a patient with an acute type B dissection, the situation becomes extremely grave [10,22,23]. When such complications occur, the patient is.usually taken immediately to the operating room or dies before surgical intervention can be undertaken [12,23]; emergency surgery in these circumstances can be associated with a mortality rate exceeding 75 percent [23]. In our 1979 report (containing 20 patients with acute type B dissections) the presence of one or more complications relating to the dissection per se elevated the operative mortality risk to 73 percent, compared to 11 percent for patients without any complications (p = 0.009) [10]. Thus, even though approximately 67 to 80 percent of medically treated patients will survive the initial hospitalization [13], if treatment fails or a major complication ensues, the patient's prognosis is part passu transformed into one of high risk [10,23]. It therefore seems ironic and perplexing that the indications for operative intervention in many centers are exactly these, i.e., failure of medical therapy or the occurrence of major complications [11-13]. Such strategies offer little opportunity to salvage this unfortunate minority of medically treated patients with acute type B dissections.
Additional arguments that detract from the attractiveness of medical therapy for this subgroup are the facts that a finite number of patients will have contraindications to antihypertensive and negative inotropic therapy at the onset, an additional minority of patients will sustain side effects from drug therapy (e.g., azotemia) which may prompt discontinuation of therapy, and approximately 15 to 25 percent of patients initially treated successfully with antihypertensive agents will develop complications of the dissection that will eventually require surgery [30,45]. Later surgical procedures during the chronic phase of type B dissections may be associated with an even higher risk than an operation performed during the acute phase [1031,32]. That is, postponing operative intervention does not necessarily reduce the operative risk.
Furthermore, the operative mortality rate for patients with acute type B dissections (13 percent between 1976 and 1981 in our hands) is no longer significantly higher than that for patients with acute type A dissections [10,20,22,30,32-34]. Slater and DeSanctis [6] and Doroghazi et al. [12] are correct, however, in stating that patients with type B dissections in general tend to have more limited life
expectancies and higher operative risk compared to type A patients due to their age, hypertension, and associated atherosclerotic diseases.
Assuming that the patient is reasonably healthy, self-reliant, and not excessively elderly (older than 70 years of age), I favor a strategy focused on uniform, emergency surgical intervention in most patients with acute type B dissections, followed by indefinite medical treatment. If the operation is carried out prior to the onset of major complications, the operative risk is low (less than 11 percent) [10], late survival and functional results are satisfactory, and the incidence of late problems mandating reoperation is relatively low [10,29,30]. Surgeons and cardiologists alike have come to consider the technical aspects associated with this subset of dissections to be quite formidable; this stigma may simply reflect the fact that operation was not carried out prior to the onset of life-threatening complications in the past. Indeed, it is our personal experience that if planned early, operation for patients with acute type B dissections prior to the occurrence of major complications, is associated with fewer technical hazards than that for patients with acute type A dissections, for whom emergency surgery is recommended routinely [9-13,24,29,31,34,35].
In cases where the false lumen does not opacify angiographically, it can safely be assumed that the false channel has thrombosed. In these rare patients, medical management may be preferable since their prognosis is relatively good [44].
It must be reiterated, however, that firm guidelines from rigorous scientific trials concerning the most optimal form of management for patients with uncomplicated acute type B dissections are difficult, if not impossible, to derive on the basis of presently available data. Only continuing experience with routine emergency surgical treatment of these patients [10] and additional long-term follow-up of medically treated patients [12] will define the best form of therapy in this subgroup.
Chronic Type B Dissections
Indications for surgical intervention for patients with chronic type B aortic dissections are restricted and conservative, similar to patients with chronic type A dissections. Patients who develop symptoms clearly referable to the dissection and those with documented enlargement or expansion of localized saccular aneurys-mal components should be considered surgical candidates [10,41]. These patients present most frequently with upper back pain, but other symptoms due to the enlarging, space-occupying nature of these masses have been reported (see Chapter 4) [6].
As elucidated by Pressler and McNamara [42,43], the presence of a large (greater than 10 cm in diameter) saccular aneurysmal component should also be considered an indication for operation even in the absence of symptoms and documented enlargement. This is because of the high risk of rupture of such false aneurysms.
On the other hand, as was the case for patients with chronic type A dissections, there is no compelling evidence supporting surgery for patients with small (less
than 10 cm in diameter) chronic type B dissections if they are asymptomatic and no progressive enlargement of the aneurysm has been documented.
In summary, the surgical philosophy at Stanford University Medical Center is extremely aggressive for patients with acute type A or type B aortic dissections, but conservative for patients with chronic type A or type B dissections. No evidence exists in our opinion, supporting surgical resection of chronic "healed" aortic dissections which are small, which are not enlarging, and which are not producing symptoms clearly referable to the aorta.
Degenerative Ascending Aortic Aneurysms without Dissection (Marian's Syndrome)
Still another high-risk subset of patients with Marfan's syndrome and dilation of the aortic root should be discussed in this essay. Although frequently these young patients have not yet sustained an aortic dissection, their life expectancy is quite limited [46,47]. This is due in part to the high likelihood of aortic dissection or rupture of the ascending aorta. In fact, we have found localized old tears without distal propagation of dissection in several of these cases even when the situation was not appreciated angiographically. These chronic cases may be the only true clinical examples of what has been termed DeBakey type II "dissecting aneu-rysms" [17]. Such a patient is presented in Figure 8-3. McDonald and colleagues
from Johns Hopkins Hospital have recently made an important contribution concerning the management of these patients [46]. Their large, closely followed population of patients with the Marfan syndrome (47] has allowed these investigators to suggest that elective surgical intervention is preferable and prudent in these patients if the ascending aortic diameter is greater than 5.5 cm in diameter
(by echocardiography), even if the patient is asymptomatic and no evidence of enlargement exists. Given the low operative mortality rate (no deaths among 13 patients in McDonald's report) [46] and 2 percent in 85 patients-(1964 to 1981)—with degenerative ascending aortic aneurysms in out hands) and the authors' knowledge of Ac national history of these patients [47], this argument is persuasive.
DIAGNOSIS AND PERIOPERATIVE MANAGEMENT
Both the diagnosis and medical therapy of patients with aortic dissections are covered exhaustively in other chapters of this monograph, but a few salient concepts pertain uniquely to the context of surgical treatment and will be discussed here.
As emphasized explicitly throughout this book, the key to future improvement in the salvage rates for patients with aortic dissections is an increased clinical index of suspicion and more prompt recognition and treatment. We [36,39,48] and others [24,28] have employed newer imaging modalities which appear to offer enhanced diagnostic precision and safety. CT scanning is now a firmly established diagnostic technique for patients with aortic dissections; it offers rapid, safe, and conclusive confirmation of the presence or absence of aortic dissection (see Chapter 6) [24,28-36]. Emergency CT scans performed in smaller, geographically remote hospitals which do not have cardiac surgical capability can provide prompt confirmation of the diagnosis. The patient can then be swiftly transferred by air ambulance to facilities where emergency aortography, intensive perioperative medical treatment, and definitive surgical intervention can be carried out with dispatch. CT scanning has also yielded important postoperative information [24,28,36]. It is employed to delineate the patency status of the false lumen and to evaluate serially any progression of the initial dissection, redissection, or formation of localized saccular false aneurysms in the distal thoracic or abdominal aorta. Further, CT scanning is useful in following medically treated patients with chronic dissections in an attempt to detect the formation of aneurysms before they rupture.
The new technique of digital subtraction angiography (DSA) promises to yield even more important information. Performed using either lined-scanned [39] (Figure 8-4a) or area-image [48] (fluoroscopic) (Figure 8-4£) radiological and computer techniques, an intravenous injection of contrast medium can result in satisfactory imaging of the aorta and the peripheral arterial tree. Although the resolution of DSA is less than that of conventional in tra arterial catheter angiograms, it is adequate enough to make the diagnosis of aortic dissection in most cases. In addition it is safe, rapid, and may eventually offer unique physiological information concerning the differential blood flow dynamics in the true and false lumens and the perfusion of important distal tributaries. Postimage processing using a coupled digital computer may enable selective visualization of solely the false lumen or solely the true lumen, yielding accurate determination as to which aortic branches are supplied by which channel. Videodensitometric (computer-assisted) systems may measure quantitive or relative flow rates in each channel.
Two-dimensional echocardiography can diagnose patients with type A aortic dissections [49], but the general utility, specificity, and sensitivity of this technique remain to be clarified (see Chapter 6). In the future, computer-reconstructed
three-dimensional echocardiographic images may be more useful.
Cinefluoroscopic techniques commonly used for coronary arteriography have been applied to evaluate aortic dissections (see Chapter 5) [50]. This technique appears to offer superior pathoanatomic information about aortic dissections, since the "real time" images can depict motion of intimal flaps and the timing of flow in the true and false channels; (this may also be true in the near future for computer-enhanced DSA techniques.) The biplane capability offered by cine-fluoroscopy also yields additional information with less radiation exposure compared to the traditional large (cut film) techniques. Such information may help the surgeon plan the operative approach more intelligently. Future widespread implementation of CT scanning, DSA, and possibly three-dimensional echocar-diographic techniques as screening tests will hopefully result in earlier recognition and diagnosis, which should translate into improved survival rates. The overwhelming importance of this need for more rapid diagnosis of patients with acute dissections is emphasized in a recent publication from Canada [51]. Almost one-half of patients with an acute aortic dissection admitted to one particular hospital were not correctly diagnosed until 24 h had transpired. During this interval, these misdiagnosed, untreated patients were exposed continuously to a risk of sudden death which approached 3 percent per hour [51]. Thus, it is extraordinarily clear why more emphasis should be placed on prompt recognition and diagnosis of these patients if the overall mortality rates are to be reduced in the future. Furthermore, the relatively large number of patients with chronic type A dissections seen today may imply that upward of 50 percent of patients with acute type A dissections are simply sudden death victims, whose unexpected demise has been attributed to tachyarrhythmia or myoc.irdi.il infarction in the absence of an autopsy. Thus, the true incidence of acute aortic dissection may be several times higher than we currently believe.
Angiographic Surgical Planning
In the past, catheter aortography has been necessary to confirm the diagnosis, determine the type and other pathoanatomical features of the dissection, decide future therapy, and enable the surgeon to plan his or her approach (see Chapter 5). The aortography is performed prior to operation whenever the patient's condition permits, but as Meng and colleagues [35] have pointed out, it is not absolutely mandatory if the clinical status of the patient is precarious. Aortography can almost always reveal whether the ascending aorta is involved in the dissecting process, but it frequently cannot discern with certainty the actual site of the primary intimal tear. It should also be remembered that in 5 to 10 percent of cases, a tear cannot be identified at the time of postmortem examination [2]. Confusion may also arise when multiple large fenestrations between the two channels are seen on the aortogram. These factors represent another distinct advantage of the Stanford classification system. While we prefer to perform aortography prior to operation in all cases, DSA and dynamic CT scanning technology in the future may replace the need for catheter angiography.
In patients with acute dissections, we usually perform additional (biplane) injections to define the anatomy of important aortic branches, e.g., arch vessels
and the superior mesenteric, renal, and iliac arteries. This is not pursued if the patient is clinically unstable or has impaired renal function. Coronary arteri-ography is technically difficult and can be hazardous in patients with acute type A dissections, and therefore it is usually not performed. Intraoperative assessment of the coronary arteries determines whether or not special adjuncttve measures are necessary to restore the integrity of the coronary circulation.
For patients with chronic dissections, more extensive angiography or DSA is performed and frequently is combined with dynamic CT scanning. A detailed assessment of all major aortic tributaries is helpful. If necessary, complete (intra-and extracranial) cerebral arteriography is carried out the next day. In cases of chronic type A dissections with aortic regurgitation, complete left and right heart catheterization and coronary angiography are usually performed as one procedure. Arguments supporting complete coronary arteriography for patients with chronic aortic dissections are based on different grounds: If the chronic dissection is type A, it is important for the surgeon to know whether the coronary arteries have been compromised or occluded by the dissection or whether coexistent atherosclerotic coronary artery disease is present in order to plan the operative procedure more completely. If the patient has a chronic type B dissection, the likelihood of coexistent atherosclerotic coronary artery disease is high due to the risk factors and age associated with this patient subset [1,2,6,10,12]. It is helpful to know whether severe three-vessel or left main coronary disease exists since this knowledge may occasionally prompt a two-stage surgical approach: myocardial revascularization followed by resection of the descending aortic dissection.
Perioperative Medical Management
Preoperative Care As outlined above, expedient diagnosis and treatment are essential in cases of acute dissection. When a referring physician contacts our cardiovascular surgical unit and arrangements are made to transfer a patient, a
series of diagnostic and therapeutic steps are immediately organized and coordinated to eliminate delay once the patient arrives. The emergency room, cardiac intensive care unit, angiography suite, and operating room are alerted, and the cardiac anesthesia and cardiovascular radiology staffs are assembled. As soon as the patient arrives, the cardiovascular surgical or anesthesiology team inserts the appropriate monitoring lines and draws the necessary blood samples in the emergency room. The patient is then taken immediately to the angiography suite. We usually monitor the ECG, CVP, arterial pressure, and urine output (with a Foley catheter). If the patient is not hypotensive, intravenous sodium nitroprus-side and/or trimethaphan camsylate drips are started; incremental doses of pro-pranolol are also given intravenously (see Chapter 7). Mean arterial pressure is kept in the range of 55 to 70 torr unless such blood pressure levels result in oliguria, depressed levels of consciousness, or myocardial ischemia. If the diagnosis of acute type A (all patients—see above) or acute type B (most patients—see above) is confirmed by aortography, the patient is taken directly to the operating room. Eliminating the usual delays encountered in the emergency room and in
transferring the patient from the emergency room to the ICU, to the angiography suite, back to the ICU, and finally to the operating room markedly streamlines the process and saves precious hours. Continuous monitoring and assessment of the patient is assured by virtue of constant anesthesia support. If, however, the patient's vital signs deteriorate, he or she is taken immediately to the operating room without the benefit of angiography for emergency surgical treatment. Assuming that the patient remains stable, intravenous beta blockade is gradually titrated upward and the intravenous vasodilator drips regulated accordingly throughout this preoperative phase. Anesthetic induction is undertaken paying critical attention to blood pressure and to determinants of myocardial oxygen supply and demand, using intravenous narcotics (currently fentanyl) and diazepam supplemented judiciously with small amounts of inhalational agents to control blood pressure and myocardial contractility.
Postoperative Care The postoperative surgical management of these patients
varies only slightly from our routine cardiac surgical protocols. Careful attention to blood pressure control is essential; we continue the same nitroprusside and/or trimethaphan camsylate IV drips as used preoperatively, supplemented judiciously with small IV doses of propranolol. This optimizes the myocardial oxygen demand and supply ratio, reduces the amount of postoperative hemorrhage, and minimizes the threat of acute redissection, aortic rupture, or disruption of the surgical anastamoses. These antihypertensive and negative inotropic drugs are weaned slowly, and alternative IM, IV, and eventually oral medications are gradually introduced as the patient's condition improves. The prophylactic antibiotics (nafcillin sodium and/or cephamandol) are discontinued after 36 to 48 h.
If inadequate postoperative perfusion of an important end organ (e.g., kidney or small bowel) is suspected, immediate diagnostic steps must be taken. A portable gamma camera is used in the ICU to evaluate renal blood flow using radionuclide techniques. DSA or conventional arteriography are occasionally necessary in the immediate postoperative hours or days. If important oligemia or ischemia is confirmed, then appropriate peripheral vascular reconstruction should be performed promptly. These life-threatening complications arise most commonly following operative repair of chronic dissections (see Figure 8-2), but can occur in patients with acute dissections. Rarely, it may be necessary to perform a surgical "fenestration procedure" in the aortoiliac system to restore normal distal perfusion.
Postdischarge Guidelines All patients who undergo implantation of synthetic
vascular prosthetic grafts on our services receive antiplatelet agents for 6 months. Our current regimen calls for sulfinpyrazone (200 mg qid), but low-dose aspirin (2% grains once daily) or aspirin and dipyridamole (5 grains acetylsalicylic acid qd and 75 mg DPM qid) may be equally effective in increasing the shortened platelet survival that occurs in patients with Dacron arterial grafts. It is very important to educate the patient and the family regarding adequate prophylactic antibiotic use to prevent prosthetic endocarditis and graft infection. Antibiotic prophylaxis
is absolutely mandatory for an indefinite time, whether the patient has a prosthetic valve and aortic graft, or simply a synthetic aortic graft.
As discussed elsewhere in this chapter, indefinite good medical follow-up and treatment with antihypertensive and negative inotropic drugs is of cardinal importance in the combined surgical and medical management of patients with aortic dissections; these issues are discussed more completely in other chapters. I believe it is prudent to prescribe beta-blocking agents indefinitely to all patients who have undergone surgery for an aortic dissection, even if they are not hypertensive. By reducing aortic shear stresses and dpldt, this therapy may reduce the incidence of late postoperative problems, including acute redissection. This philosophy is totally empirical, and proof of its efficacy has not been addressed; such studies are needed. Nevertheless, it seems reasonable that once-a-day oral treatment with the newer beta-blocking agents is well worth the small amount of inconvenience, risk, and cost, since it appears to improve the long-term postoperative prognosis of these patients.
OPERATIVE TECHNIQUES
Cardiopulmonary Bypass
It is our policy to use cardiopulmonary bypass (CPB) with pump oxygenation for patients with type A as well as those with type B dissections, but alternative methods for patients with dissections involving the descending aorta have been advocated [31]. As illustrated in Figure 8-5a, total CPB (similar to that employed for most open-heart procedures around the world) is used for patients with type A dissections. The principal difference is that the arterial inflow from the pump is routed to one of the common femoral arteries rather than to the ascending aorta. Peripheral (femoral) arterial cannulation is preferred over central (aortic) cannulation due to the hazards associated with cannulation of the dissected, double-lumen ascending aorta. If known angiographically, a femoral artery perfused by the true lumen is selected for cannulation; if this information is not known, then either femoral artery can be used, but it is incumbent upon the surgeon to assess quickly the adequacy of pump arterial inflow when CPB is commenced. Venous drainage is achieved via two (28 French) caval catheters inserted via the right atrium.
Total cardiopulmonary bypass implies that the pump oxygenator is responsible for systemic blood flow to the entire body and oxygenation of this blood. Thus, pulmonary blood flow is nil and the lungs do not need to be ventilated. Our standard total perfusion techniques entail low-flow (2000 to 3000 cc/min), low-pressure (30 to 60 torr) cardiopulmonary bypass and modest systemic hypothermia (29 to 31° C). These perfusion rates and pressures are lower than those usually targeted at other institutions; additionally, many centers cool the patient more profoundly (in the range of 22 to 24° Q. During the period of aortic cross-clamping ("ischemic arrest"), myocardial protection is afforded by single-dose (500 to 1000 cc) cold hyperkalemic cardioplegia and continuous profound topical hypothermia.
In contrast, partial CPB implies that the pump oxygenator is supplying the demands of only part of the body for oxygenated blood. Femoral-femoral partial CPB is used at Stanford for patients with type B dissections, and it is imperative that the lungs continue to be ventilated. As shown in Figure 8-56, partial CPB involves venous drainage from the right atrium (via a long cannula inserted in the common femoral vein) and femoral artery pump inflow. If venous drainage is inadequate or the caval cannula cannot be passed beyond the common iliac
vein-inferior vena cava junction, another " Y-ed" venous cannula can be inserted through a purse-string suture into the main pulmonary artery to supplement venous return. Once the descending thoracic aorta is clamped, the pump oxygen-ator takes a portion of the systemic venous return from the heart (1000 to 1500 cc/min), oxygenates this blood, and pumps it to the abdominal and lower thoracic aorta (including the contralateral leg). The remainder of the venous blood follows the natural circulation, is oxygenated by the lungs, and is pumped to the proximal body (coronaries and arch vessels) by the left ventricle. Systemic cooling is not used during partial (femoral-femoral) cardiopulmonary bypass in our institution.
A pulmonary artery monitoring catheter is useful to control the proximal arterial pressure and to ensure adequate decompression of the left ventricle on partial CPB. The perfusionist is given a targeted range of pulmonary artery dias-tolic (PAD) pressure once the patient is positioned in the right lateral decubitus position. During CPB, the perfusionist tracks this PAD pressure (by adjusting the amount of systemic venous blood returning to the pump oxygenator) instead of tracking the proximal arterial pressure (usually the right radial artery) by adjusting the flow rate. A relatively constant pump flow is thereby maintained. The perfusionist is effectively tracking an "active" parameter (estimate of left ventricular filling pressure) instead of a "passive" parameter (proximal arterial pressure). This approach eliminates the dangerous, wide swings in proximal arterial pressure and left ventricular end-diastolic pressure which can occur otherwise. Left ventricular filling is adjusted to keep the proximal mean arterial pressure in the range of 70 to 80 torr, using supplementary sodium nitroprusside if necessary. Distal body arterial pressure (pump-generated flow) is not monitored in all cases (dorsalis pedis arterial line), but can be informative. The pump flow rate is maintained at 1 to 1.5 liters per minute.
The use of selective bronchial intubation (currently using polyvinylchloride double-lumen endobronchial tubes instead of the older Carlens' and Robert-Shaw tubes) markedly facilitates operations on the descending thoracic aorta. The left lung is selectively deflated during the procedure which enhances the surgical exposure and minimizes trauma to the lung. A tube large enough to ensure adequate bronchial suctioning must be used and arterial blood gases (obtained from the radial artery) must be checked frequently to ensure that adequate ventilation is being maintained.
Acute Type A Dissections
With the heart and great vessels exposed via a median sternotomy, the damage inflicted by the acute dissection is readily apparent. As shown in Figure 8-6a, the ascending aorta is ecchymotic. The dissection can extend under the adventitia of the pulmonary artery, past the epicardium over the atria, as far as the right ventricle such that the entire heart appears ecchymotic. Bloody fluid is frequently found in the pericardial sac even if frank rupture has not occurred. The adventitia containing the dissection is very thin (Figure 8-66). Figure 8-7 demonstrates a
typical transverse intimal tear located just above the aortic commissures. Distal fenestrations have the same general appearance.
The ascending aorta is opened obliquely, and the extent of damage to the coronary arteries and the aortic valve is assessed (Figure 8-8). An estimate of whether the natural valve can be preserved is made at this point. In our experience, preservation of the natural valve has been possible in 84 percent of patients with acute type A dissections. This is possible in acute cases because the valve per se usually is not damaged, merely prolapsed (Figure 8-9). It also must be decided whether concomitant coronary artery bypass grafting is necessary to reconstitute coronary flow. If the valve can be spared, the aorta is transected circumferentially just above the commissures (Figure 8-86). Exquisite care must be taken in dis-
secting out a full-thickness cuff of proximal aorta diligently conserving aortic adventitia. The decision to use strips of Teflon felt as bolsters is made on an individualized basis depending on the integrity of the tissue encountered. Occasionally two layers of Teflon felt are necessary [40]: one strip (tailored to accommodate the coronary arteries) between the intima and adventitia in the false lumen and another externally (Figure 8-8c). Frequently, only one external layer of Teflon felt is needed, or, occasionally, none at all. The full-thickness aortic cuff is then reconstructed using a running 4-0 SHI Prolene' suture, as illustrated in Figures 8-8dand 8-lOa. The aorta is then circumferentially transected distally and a similar cuff reconstruction performed, but a middle layer of Teflon felt is not necessary here (see Figure 8-13J). The bulk of the dissected aortic wall (including the primary intimal tear, if exposed) is resected. If the aortic wall is densely adherent to the surrounding structures (specifically the right and main pulmonary arteries), no attempt is made to completely resect the entire aneurysm: Extreme care and gentleness must be taken in reconstructing these aortic cuffs such that the intima and/or adventitia is not torn by passage of the suture needles. If such occurs, troublesome bleeding may occur externally and, more importantly, blood may escape through the needle holes from the true lumen into the false lumen.
The proximal graft anastomosis is constructed using a second 4-0 SHI or 3-0 SH Prolene suture (Figure 8-8e) taking full-thickness bites incorporating all layers of the aortic cuff (Figure 8-106). As emphasized before, for the first suture line, care is necessary to prevent tearing of the fragile tissues with each passage of the needle.
Our synthetic conduit of choice for these cases is a soft, low-porosity woven Dacron graft.2 The extreme low porosity of this graft eliminates the threat of intraoperative hemorrhage through the interstices of the graft, whether or not the graft is pre-clotted. Completion of the distal anastomosis to the previously prepared aortic cuff is then performed (Figures 8-8/and 8-11) using 3-0 or 4-0 Prolene.
If the aortic valve is irreversibly damaged, if it is bicuspid, or if it cannot be resuspended adequately, concomitant aortic valve replacement (AYR) is carried out. Two basic alternative and, possibly, complimentary surgical techniques are
commonly used today: the "conventional" method of separate AYR and ascending aortic graft insertion (which I have favored) [52] and the "composite" method which employs a prefabricated valve-graft conduit and requires side-to-side coronary artery anastomoses [53]. As shown in Figure 8-12, a substantially greater proportion of the sinuses of Valsalva can be excised if AYR is necessary compared to when the patient's natural valve is preserved (Figures 8-8c, d, and h). Small tongues of aortic wall surrounding the coronary ostia are retained, and the proximal graft is tailored appropriately (Figure 8-8g).
In our experience, the natural aortic valve can be preserved in 84 percent of cases of acute type A dissections. If the patient has the Marfan syndrome or annuloaortic ectasia, however, concomitant valve replacement should always be performed to preclude subsequent development of aortic regurgitation due to the preexistent annular dilatation and attenuation of the valve leaflets [10,52]. Some surgical authorities have recommended universal AYR in cases of acute type A dissection [54], but we [10,52] and others (13,24,35,40] have found that a philosophy of selective AYR yields good long-term function of the patient's natural valve without the additional long-term risk imposed by a prosthetic or bioprosthetic artificial valve.
Chronic Type A Dissections
The operative techniques previously described for cases of acute dissection are similar to those used for patrtnts with chronic type A dissections. Exceptions
include the less frequent need to use Teflon felt supporting bolsters since the tensile strength of the aortic tissue is distinctly greater in the chronic phase [10]. Another important difference is the fact that concomitant aortic valve replacement (AYR) is necessary more frequently (45 percent of our patients) in chronic cases with aortic regurgitation [10,13]. This is due to the extent of chronic valvular damage and the unlikelihood that simple commissural resuspension will eliminate the valvular regurgitation.
One surgical question concerns whether the two lumens should be obliterated at the distal graft anastomosis. It has been our routine practice to do so [10], but if a large disparity exists between the circumferences of the two lumens, the relatively stiff, immobile nature of the adventitial layer makes this a technically difficult task. Additionally, the distal false lumen remains patent in over 50 percent of cases [28,36-40], even if obliteration of antegrade flow at the distal anastomosis is attempted. As mentioned above, this is probably due to large distal reentry points or fenestrations (Figures 8-20 and b). Furthermore, if an important distal aortic branch (e.g., superior mesenteric or renal artery) has become totally dependent on the false lumen for perfusion (illustrated in Figure 8-2/) and obliteration of all antegrade flow into the false lumen is technically successful, immediate postoperative renal, visceral, or spinal cord ischemia can occur and prove to be fatal. These arguments have prompted the University of California at San Francisco cardiac surgical group to redirect purposefully distal graft flow into both the true and false lumens in certain patients [28]. This concept is illustrated in Figure 8-13 and is accomplished by partially resecting the aortic intima or "septum." Since the redistribution of distal blood flow after operation in cases of chronic dissection is unpredictable, this maneuver may prove to be prudent in some cases despite the theoretical appeal of attempting to obliterate the false lumen in order to hasten thrombosis in this channel. Which individual patients will benefit from this
modification is still undefined, and corroboration of the efficacy of this technique is needed. It must be emphasized that this approach which maintains antegrade false-lumen flow applies only to patients with chronic dissections, and should not be confused with the surgical approach in patients with acute dissections.
As described in the section on acute type A dissections, two alternative technical concepts are currently employed for concomitant AYR and replacement of the ascending aorta [52-53]. We have favored the conventional technique (Figure 8-14) over the composite because of its simplicity and good long-term results in our hands [52]. Also, concern exists regarding the possible occurrence of coronary artery false aneurysms with the composite technique [55]. These false aneurysms can develop after the composite procedure at the side-to-side anastomoses between the coronary ostia and the Dacron graft, but the incidence of such potential complications is not currently known because all patients have not undergone late postoperative aortography and follow-up of patients undergoing the composite procedure is relatively short [53]. The University of Minnesota group has recommended routine late aortography for all patients undergoing the composite procedure to detect any occurrence of false aneurysms [55]. Conversely, the major potential disadvantage of the conventional method is possible late recurrent formation of aortic root aneurysm between the proximal graft anastomosis and the aortic annulus.
We at Stanford have used the composite procedure only rarely and usually only
when the coronary ostia are displaced far (3 to 7 cm) cephalad from the aortic annulus. This latter feature is a not uncommon frequent finding in patients with annuloaortic ectasia or Marfan's syndrome. To obviate the possibility of the occurrence of late coronary false aneurysm, we believe the coronary arteries should be reimplanted as full-thickness end-to-side anastomoses using the Carrel patch technique (Figure 8-15) [52]. Although more time consuming, the use of this technique effectively eliminates the possibility of anastomotic false aneurysm. More of the sinuses of Valsalva can be resected if the coronary arteries are reimplanted.
Using the conventional technique, the annulus is ringed with interrupted horizontal mattress Ethibond sutures after the diseased valve is excised, as illustrated in Figure 8-14o. The bioprosthesis is then seated and the sutures are tied. The bulk of the diseased aortic root is excised preserving only a 3- to 5- mm rim above the annulus and the coronary ostia. This produces small tongues of aortic wall around
right) are displaced far distally, they are dissected out as full-thickness Carrel "buttons" and reimplanted (Figure 8-15). The proximal aortic cuff is then prepared for anastomosis using a running Prolene suture to obliterate the false lumen. The distal circumferential full-thickness aortic cuff is then dissected out and prepared similarly. Although many surgeons do not feel that it is necessary to completely transect the aorta and suture the graft to a circumferential full-thickness cuff of aorta [34], we feel that this technique is preferable to reduce the incidence of postoperative anastomotic false aneurysms. This technique also facilitates suture control of any anastomotic bleeding points under direct vision, in contrast to other techniques [34,53] which wrap the graft completely with the residual (aneurysmal) aortic wall to aid hemostasis. The large majority of the diseased aorta is completely excised. Operative examples are shown in Figure 8-16.
In chronic cases, it is necessary to reconstruct contiguous vessels more frequently than in acute cases. This can involve coronary artery bypass grafts, graft replacement of the innominate artery, and replacement of portions of the aortic arch, as illustrated in Figure 8-17. Preoperative selective coronary arteriography is safer and less technically demanding in patients with chronic type A dissections compared to those with acute dissections, and the information it provides can be extremely helpful. Whenever it is technically possible, preoperative coronary an-giography is carried out in these patients.
Aortic Arch Dissections
This surgical topic remains poorly characterized due to its rarity, the absence of large studies, and the multitude of surgical techniques used. The optimal management of patients with acute arch dissections is not known, but improved surgical results have recently been published [57]. Two principal alternatives exist for protection of the brain during resection of the aortic arch: profound systemic hypothermia and circulatory arrest versus CPB perfusion methods. Griepp [57], Crawford [58], and Cooley [59] have reported promising early survival statistics using circulatory arrest, but we continue to use a simplified cardiopulmonary bypass (CPB) system using moderate (26 to 30° C) hypothermia in most cases. Preoperative selective cerebral arteriography is carried out to determine if the
circle of Willis is intact and to document the presence of any associated extra-cranial or intracranial atherosclerotic disease. In addition, the adequacy of cross-cerebral collateral perfusion is assessed using temporary balloon-catheter occlusion of the left common carotid artery [60]. This Enzmann balloon catheter3 enables the awake patient to be observed during temporary carotid occlusion and the distal cerebral "stump" pressure and extracranial-intracranial flow dynamics to be measured. If the patient tolerates 5 min of unilateral carotid occlusion without developing any neurological deficit, then we believe it is safe to perfuse only one carotid vessel during resection of the aortic arch. Hence, only a simple one-arterial pump-head CPB system with the arterial inflow line "Y-ed" into one femoral artery and the innominate artery is sufficient (Figure 8-18).
The median sternotomy incision can be extended into the neck if necessary, and complete dissection of the ascending aorta, arch vessels, and proximal descending aorta is carried out. Cardiopulmonary bypass is commenced and the patient is cooled to a modest degree. The innominant, left common carotid, and left subclavian arteries are clamped* Distal control is obtained either using an in-traaortic Fogarty aortic occlusion balloon catheter or a conventional vascular clamp. The aortic cuffs are prepared as described above, and the distal anastomosis is performed first. The three arch vessels are then dissected out as a button of aorta, the dissected aortic wall is reconstructed with a running Prolene suture, and the
button is reimplanted into an oval opening in the top of the graft. After flushing of air or any retained paniculate matter from the graft and arch vessels, the distal clamp (or balloon) is removed. The graft is then clamped proximally and pump flow via the femoral cannula thereafter perfuses both carotid arteries. The proximal anastomosis is carried out next, with restitution of coronary blood flow following removal of the aortic cross-clamp.
Acute Type B Dissections
A left posteriolateral thoracotomy is used for operations involving the descending thoracic aorta. The area of most severe aortic damage is identified, being usually located just distal to the left subclavian artery. Proximal aortic control is accomplished between the left common carotid and the left subclavian arteries. The length of aorta replaced is designed not to be extensive in order to minimize the number of intercostal arteries that must be sacrificed. This is done to reduce the incidence of paraplegia. As shown in Figure 8-19, after institution of partial femoral-femoral CPB and deflation of the left lung, the proximal descending aorta is opened longitudinally. If exposed in this segment of the aorta, the primary intimal tear is resected. If the tear is located in the distal arch between the carotid and subclavian arteries (and there is no retrograde propagation which would make this a type A dissection), the proximal aortic cross-clamp can be placed just distal to the innominant artery to facilitate resection of the intimal tear. Any bleeding intercostal arteries are suture-ligated from within the aortic lumen (Figure 8-196). Full-thickness proximal and distal aortic cuffs are then dissected out and prepared as described above. External Teflon felt strips are used frequently, but in contrast to acute type A dissections, it is rarely necessary to use a second Teflon felt strip inside the false lumen for additional support. Exquisite care is necessary in the preparation of these cuffs and in obliteration of the false lumen to avoid troublesome bleeding and possible disruption of the graft anastomosis since these tissues are extremely fragile. The medial portion of the dissected aortic wall is not resected, but simply left in situ. The graft thereby lies inside the old aneurysmal aortic wall (Figure 8-20).
The proximal and distal anastomoses are performed sequentially. After ensuring that all air and retained particulate matter are flushed out of the graft before completing the distal anastomosis, the clamps are removed, the anastomosis finished, and the area policed for hemostasis. Partial CPB can then be rapidly discontinued and the heparin effect reversed with protamine sulfate.
Chronic Type B Dissections
The operative techniques employed for patients with chronic type B dissections are similar to those already described (Figure 8-21). The same question concerning whether to perfuse solely the distal true lumen or both the true and false lumens applies to these patients as well as to those with chronic type A dissections [27]. As outlined above, this is not an issue in cases of acute dissections.
The longitudinal extent of aortic replacement is arguable. The Baylor group is currently advocating extensive replacement of the descending thoracic aorta, including the entire thoracoabdominal aorta in certain cases [29], but our approach limits the length of the aorta replaced to include only the fusiform or saccular segment containing the most severe damage Although the entire descending thoracic aorta is dissected and aneurysmal to varying degrees in cases of chronic type B dissections, it is frequently only a relatively localized segment that is expanding or causing the patient's symptoms. Thus, we replace only a conservative segment of aorta; this practice has been successful in limiting the incidence of operation-induced paraplegia (no cases in 25 patients using partial CPB), but it does have the theoretical disadvantage of leaving the patient with a larger amount of diseased aorta, which can potentially expand and rupture [29,30]. Judgment and experience are necessary, here, as it is unrealistic to expect any method of intraoperative spinal cord protection (femoral-femoral CPB, heparinized temporary shunts, simple cross-clamping alone, hypothermia, left atrial-femoral bypass, etc.) to proffer total immunity from paraplegia.
Newer Operative Techniques
Two recent technical innovations merit discussion. The Foch-Universite cardiovascular surgical group from Suresnes, France, has used a gelatin-resor-
cine-formol (GRF) biological glue since 1977 to reinforce the tissue integrity of the aortic cuffs. When compared to a noncurrent cohort of 25 patients operated upon using more conventional techniques, they showed both improved early and late results [61]. Additional research studying this material and other biological adjuncts (fibrin adhesives) [62] may offer important advances in the future in the surgical treatment of acute aortic dissections.
In an attempt to reduce the incidence of serious intraoperative hemorrhage following anastomosis of a graft to a dissected aorta, several groups [63-65] have used a ringed intraluminal prosthesis. These Dacron tubes are inserted intOxthe proximal and distal aortic true lumen and secured with special tapes encircling the aorta. This technique is most applicable to type B dissections, which constitute a minority of patients [1,2,10,12,13,23,24,27,44]. Due to small numbers of patients and only limited follow-up reports, average aortic cross-clamp times in the same range as for conventional techniques [65], and concern regarding prosthesis migration, aortic wall necrosis and erosion, false aneurysm formation, and compromise of aortic commissural suspension and/or coronary ostial patency, I believe that any putative advantages of this technique still remain to be proved. Larger comprehensive investigations encompassing longer follow-up are necessary before meaningful comparison with the current methods (associated today with mortality rates for patients with acute dissections ranging from 8 to 13 percent [24,34,35] can be undertaken.
RESULTS OF SURGICAL TREATMENT
A complete review of the results of therapy, both medical and combined medical and surgical, is presented elsewhere in this monograph. Therefore, this essay will confine itself to a description of the surgical results, with emphasis being given to the more recent achievements at Stanford and elsewhere. This includes a new, comprehensive update of our experience at Stanford with a total of 162 consecutive patients treated surgically.
Standards In the Literature
When we presented our observations of surgical treatment of aortic dissections over a 16-year interval (February, 1963 through January, 1979) in 1979 [10], a compendium of the reported surgical results since 1970 was used as a literature benchmark. These results, including our 1979 report [10], are shown in Table 8-1. The patient populations in each report have been reclassified, where possible, to conform with the Stanford type A and B classification system. Therefore, the results summarized in Table 8-1 do not correspond exactly to the original numbers presented.
The average operative mortality rate for patients with acute type A dissections was 37 percent (86 in 232), and ranged from 18 percent to 100 percent. For patients with chronic type A dissections, the average mortality rate was 17 percent (18 in 103) (range = 0 to 67 percent). For patients with descending or type B dissections, the surgical results in this older time frame were similar: 38 percent of 141 patients with acute type B dissections (range 0 to 75 percent) died as did an average of 24 percent of 95 patients with chronic type B dissections (range 0 to 50 percent). It is noteworthy that the center with the highest operative mortality rate (75 percent) for patients with acute type B dissections operated only on patients who had failed acute medical treatment [23]. Conversely, the Texas Heart Institute's low operative mortality rate of 25 percent for patients with acute type B dissections would have been only 12 percent had 6 (of the 12) fatalities who turned out to have retrograde extension of the dissection (i.e., effectively having type A dissections) [27] been excluded. It also must be pointed out that these overall results included patients undergoing operation over a 10- to 20-year interval, dating back to 1963 in some series [10].
These surgical results were also compared with reported hospital mortality rates with medical treatment. The average surgical mortality rate of 37 percent for patients with acute type A dissections was markedly superior to the 83 percent mortality rate for 76 patients treated medically [10]. Seven of the series included medical patients with acute type B dissections; among these 67 patients the average hospital mortality rate was 33 percent, compared to the average surgical mortality rate of 38 percent. For patients with chronic type A or type B dissections, the hospital and operative mortality rates were quite similar, but in contrast to their medical cohorts, the patients treated surgically had appropriate symptoms, an expansion of the dissection, or some major complication, including frank aortic rupture.
In the Stanford series of 125 unselected patients, several determinants portending increased operative risk were elucidated {10]. Focusing on 12 preoperative complications due to the dissections per se, the operative mortality rate for 42
patients without one or more of these complications was 14 percent compared to 36 percent for the remaining 83 patients (p = < 0.05). This difference in outcome was particularly dramatic for patients with acute type B dissections: Only 11 percent of 9 patients without any major complications died in contrast to 73 percent of 11 patients with one or more of these complications (p » 0.0009). Three of these 12 complications had a pronounced bearing on operative outcome: stroke, paraplegia, and angiographic evidence of renal and/or visceral ischemia. The operative mortality rate was 24 percent for 106 patients without one or more of these three complications versus 58 percent (p < 0.05) for the remaining patients who had one or more of these three complications.
In this series of 125 patients, the primary intimal tear was located in the ascending aorta in only 84 percent of patients with type A dissections; it was in the aortic arch in 10 percent (Figure 8-lc) and in the descending aorta in 6 percent of cases (Figure 8- If). Similarly, the tear was located in the descending aorta in only 77 percent of patients with type B dissections, being in the arch in the remaining 23 percent of cases (Figure 8-W). Since the operative procedure did not entail resection of the primary intimal tear unless it was exposed in the aortic segment being replaced, the actual tear was not resected in 22 percent of cases. Analysis of operative mortality rates, late survival rates, late reoperation rates, and late functional results were not statistically inferior for patients without excision of the primary intimal tear. The reoperation rate, however, was (insignificantly) higher (8 percent per patient-year vs. 3 percent per patient-year) for patients without tear resection. These observations were confirmed by Turley et al. [28] and demonstrate that long-term survival in surgically treated patients is more a function of preventing rupture and other life-threatening local complications of the dissection than of eliminating all flow in the false lumen or resecting the primary intimal tear. It is our preference, however, to resect the primary tear whenever possible. This usually involves partial resection of the aortic arch contiguous with either the ascending or proximal descending aorta when the usual technique does not expose the tear. This more aggressive approach is performed more frequently today than in the past.
Late survival and reoperation were analyzed for patients with acute type A dissections depending on whether the native aortic valve had been preserved [10]. Such was accomplished in 32 surviving patients with acute type A dissections. Two of these patients later required AYR 34 and 48 months postoperatively, and two other patients had mild, asymptomatic aortic regurgitation. The two patients requiring late aortic valve replacement represented a linearized rate of 1.5 percent per patient-year; furthermore, one of these two patients had annuloaortic ectasia and, in retrospect, should have had concomitant AYR at the time of initial operation. These results suggested that the durability of operative procedures which preserve the native valve for patients with acute type A dissections was satisfactory, and have been corroborated recently by other investigators [24,35,40].
A total of 391 patient-years of follow-up was analyzed in this previous report
[10]. The overall 5-year actuarial survival rate for the 89 discharged patients was 76 ± 5 percent ( ± SEM). There was no significant difference in late survival rate among the four subgroups, but only 3 of the 11 discharged patients with acute type B dissections succumbed over an average follow-up interval of 5.1 years (63 patient-years of follow-up). When operative deaths were excluded, patients with acute type B dissections had an 85 ± 14 percent 5-year actuarial survival rate which was superior, albeit insignificantly so, to that for the other three subgroups. Inspection of the causes of late death was instructive. Forty-five percent of all late deaths were due to cardiac causes and 16 percent were related to the central nervous system. Importantly, 10 percent of all late deaths were due to late hemorrhage due to rupture of remote aneurysms. This fact is important in that DeBakey and colleagues have recently reported that 31 percent of all late deaths were due to rupture of a contiguous or remote aortic aneurysm (29]. This sobering fact makes it incumbent upon all physicians caring for these patients to follow them closely in order to detect late formation of aneurysms before rupture.
Recent Surgical Result*
Since 1979 several publications have shown improved early surgical results,
especially for patients with acute type A dissections. Cachera and colleagues from Creteil, France [24], reported an overall hospital mortality rate of 24 percent among 38 patients with acute type A dissections treated between 1971 and 1980; Meng and his associates from Chicago [35] had a 15 percent operative mortality rate in 20 similar patients between 1970 and 1978; and Wolfe [34] reported a 13 percent mortality rate among 30 patients with acute type A dissections between 1975 and 1980.
Updated Analysis of the Stanford Experience
The overall Stanford experience has been updated for the purposes of this chapter. Between February, 1963 and November, 1981 a total of 162 consecutive patients underwent surgical treatment of aortic dissections. Table 8-2 summarizes selected characteristics of this patient population; 69 had acute type A dissections, 42 had chronic type A dissections, 26 had acute type B dissections, and 25 had chronic type B dissections. Thus, 69 percent of cases were type A and 59 percent were acute', this distribution of type and acuity parallels that documented in the older autopsy reports [2,3,26], and does not represent a skewed or highly selected patient population. A total of 610 patient-years of follow-up was analyzed. Follow-up extended to 15 years maximum and averaged over 5 years. The overall operative mortality rates for each subgroup are listed in Table 8-2, but our more recent results will be emphasized (Table 8-3) in an effort to illustrate contemporary expectations and to help clarify whether medical or combined medical and surgical therapy represents the more optimal treatment today for certain patient subsets.
Contemporary operative risk-benefit guidelines can be discerned from inspection of Table 8-3. Between 1976 and 1981, 54 consecutive patients have been operated upon. Only irreversible cerebral damage constituted an absolute contraindication to operation; otherwise, no patient was denied surgical treatment because of age, complications of the dissection, or moribund status.
For 24 patients with acute type A dissections, the recent operative mortality rate was 8 percent, which represents a distinct improvement over an earlier experience (38 percent mortality rate between 1963 and 1976). For patients with chronic type A dissections, the contemporary operative mortality rate was 13 percent (2 in 16). This current figure was not substantially different from the 15 percent mortality rate for the years 1963 to 1976. For patients with acute type B aortic dissections, the current operative mortality rate was 13 percent (1 in 8), dramatically lower than the 50 percent mortality rate for the earlier 13-year period. This pronounced decline reflected ongoing improvements in surgical, anesthetic, medical, and nursing management. It also may have been due, in part, to referral of more patients with acute type B dissections before major catastrophic complications had occurred, as the operative mortality rate in our earlier report (1963 to 1979) was only 11 percent for 9 patients with acute type B dissections who had not yet sustained one of 12 major complications (see above).
As was the case for patients with chronic type A dissections, our most recent results for patients with chronic type B dissections were only modestly better. The 1976 to 1981 operative mortality rate was 17 percent (1 in 6) for this subset
compared to 21 percent for the 1963 to 1976 period.
Updated long-term actuarial survival curves for patients in each subgroup are shown in Figure 8-22. As illustrated in Figure 8-22o, the 50 discharged patients with acute type A dissections had a 75 ± 7 percent 5-year and 49 ± 13 percent 10-year survival rate. For 36 discharged patients with chronic type A dissections, these respective rates were 73 ± 8 percent and 56 ± 11 percent (Figure 8-226). As was discussed above, discharged patients with acute type B dissections appeared to have the best long-term prognosis, although the average age (61 ±11 years) of this subgroup was the highest among the four subsets. As shown in Figure 8-22c, none of 16 discharged patients with acute type B dissection died until after 6 years postoperatively. The actuarial 5-year survival rate, by definition, would be 100 percent, but a more realistic estimate is the 90 ± 12 percent 5-year survival rate shown on the graph. The 5-year actuarial survival rate for the 20 discharged patients with chronic type B dissections was 78 ± 12 percent (Figure %-22d). We do not disagree with others who state that patients with descending or type B dissections have limited life expectancy compared to patients with type A dissections due to their advanced age and generalized atherosclerosis [6,12], but discharged patients with type B dissections had the highest 5-year survival rate in our experience. The fact that the average age of our patients with acute type A (54 ± 13 years) and chronic type A (56 ± 14 years) dissections is distinctly older than that in most reports [4,6,11,12,24,30,34,35,40,53,56,61] may explain this apparent discrepancy.
To place these survival curves in better perspective, Figure 8-23 compares the survival rate of these patients to that for a computer-simulated population matched for age and sex. The adverse impact of the relatively high overall (1963 to 1981) operative mortality rate of 23 percent for all type A patients is readily evident (Figure 8-23a), but as discussed above, this rate pertains to our total 18-year experience. As summarized in Table 8-3, our current (1976 to 1981) overall operative mortality rate is only 11 percent (6 in 54), ranging from 8 percent for patients with acute type A dissections to 17 percent for patients with chronic type B dissections. This substantial improvement will eventually neutralize the abrupt decline in survival in the first postoperative month. When operative deaths are excluded (Figure 8-236), it can be appreciated that long-term survival for surgically treated patients with type A dissections is satisfactory, but still cannot be considered optimal. Figure 8-23c focuses solely on discharged patients with acute type B dissections. It is noteworthy that the long-term prognosis for these 16 patients was statistically indistinguishable from the matched general population. Given the current operative mortality rate of 13 percent for this subset and this remarkably favorable long-term prognosis, it is difficult—if not impossible—to argue convincingly that universal surgical treatment of these patients does not represent the most optimal mode of therapy. Similarly, long-term survival for discharged patients with chronic type B dissections (Figure 8-23</) was not significantly inferior compared to the matched general population.
SUMMARY
In summary, our more recent experience between 1976 and 1981 has been punctuated by marked reductions in operative risk for patients with acute type A or acute type B dissections (Table 8-3). The 8 percent current operative mortality rate for patients with acute type A dissections compares extremely favorably with
the average hospital mortality rate of 83 percent with medical treatment reported in the literature [10], thereby strengthening the philosophy of emergency surgical treatment for these patients. Furthermore, the low current operative mortality rate (13 percent) for patients with acute type B dissections and the fact that long-term life expectancy for discharged patients with acute type B dissections in our overall 1963 to 1981 experience is identical to that for a matched, general population
(Figure 8-23c) represent compelling indirect evidence that emergency surgical intervention is also the most optimal form of treatment for this subset of patients as well.
While the operative risk for patients with chronic type A or chronic type B aortic dissections has not fallen appreciably, surgical treatment of selected symptomatic patients with enlarging or even ruptured aortic dissections is associated with reasonable operative mortality rates (13 to 17 percent), satisfactory long-term survival, and good amelioration of symptoms. The fact that the operative mortality rates for these two subsets of patients has not fallen substantially compared to our earlier (1963 to 1976) experience (in contrast to patients with acute dissections) may constitute an additional argument favoring emergency operative intervention for all patients with acute aortic dissections.
Future major breakthroughs and improvements in the overall salvage rate for patients with aortic dissections pivots on earlier recognition and prompt diagnosis. This goal will be aided by newer, safer imaging modalities, but augmented clinical suspicion and awareness must, by definition, be the mainstays behind future clinical advances. To this end it is hoped that this chapter and this volume as a whole will help serve the educational needs of Current and future physicians.
ACKNOWLEDGMENTS
The tireless, dedicated help and superb skills of Ms. Laraine Dunn in the preparation of this chapter are gratefully acknowledged and deeply appreciated. I would also like to thank Doctor Howard B. Burchell for his sagacious, insightful, and critical comments over the last 4 years pertaining to the management of patients with aortic dissections; his early pioneering contributions and his continuing efforts in this field have catalyzed many of the diagnostic and therapeutic advances described in this essay over the last 40 years.
Finally, I would like to dedicate this work to one of the most gifted and inspiring surgical teachers of all time—Dr. Norman E. Shumway.


FIGURE 8-2
Examples of distal pathoanatomic complications of aortic dissections. Perfusion of the aortic tributary is illustrated in (a), (6), and (0; however, perfusion takes place through the true channel in (a) and (b) and through the false channel in (0- (c), (d), and (e) indicate obstruction of the aortic tributary due to extrinsic compression (c) and (d) or due to compromise of the true lumen followed by subsequent thrombosis (e). In chronic dissections, the pathoanatomical situation illustrated in (f) may become permanent, such that reentry at the branch vessel has generated an intimal flap which then becomes healed to the opposite wall of the vessel. As such, this branch vessel is solely dependent on false lumen perfusion.



FIGURE 8-1
Schematic representations of the Stanford type A and type B classification system, (a), (c), and (r) The dissection is termed type A due to involvement of the ascending aorta, (c) The tear is located in the aortic arch, (f) The tear is located in the descending aorta but retrograde propagation involving the ascending aorta signifies a type A dissection. In contrast, (to) and (d) indicate a type B dissection due to absence of involvement of the ascending aorta. In a type B dissection, the tear can be located in the aortic arch, the descending aorta, or, rarely, the abdominal aorta, (e) The rare isolated aortic arch dissection in which neither the ascending or descending aorta is involved. By strict definition these are Stanford type B dissections, but probably should be referred to as isolated aortic arch dissections.


FIGURE 8-5
(a) Schematic diagram illustrating total cardiopulmonary bypass in a patient with a type A aortic dissection. Diversion of all venous blood returned to the heart via two atrial cannulas and arterial inflow into the right femoral artery are shown. The pump oxygenator is responsible for oxygenation and perf usion of the entire body. (6) Partial cardiopulmonary bypass (CPB) in a patient with a type B aortic dissection. A long venous cannula is inserted via the femoral vein and advanced to the right atrium. This diverts only a portion of the systemic venous return to the pump oxygenator. If the cannula cannot be advanced beyond the inferior vena cava-iliac vein junction or if venous return is inadequate, an additional venous line can be inserted into the main pulmonary artery (dashed lines). Arterial inflow is delivered into the femoral artery. During partial CPB the left ventricle supplies the coronary arteries and arch vessels and (after clamping of the descending aorta) the heart-lung machine oxygenates and perfuses only those organs supplied by the abdominal and lower descending aorta. Continued ventilation of the lungs is thereby mandatory.

FIGURE 8-8
Diagrammatic representation of the steps involved in the surgical repair of type A aortic dissections, (a) Limited resection of ascending aorta, (to) Primary intimal tear, both lumens, and extent of aortic resection, (c) Artist's depiction of the reconstruction of the proximal aortic group using two layers of Teflon felt. One layer is occasionally necessary in the false lumen. In almost all cases an external layer of Teflon felt (arrow) is required to bolster the tissue integrity of the fragile aorta. Note the tailoring of the Teflon felt strips to accommodate the dissected coronary ostia. (d) Reconstruction of both the proximal and the distal aortic cuffs, (e) The proximal anastomosis is started posteriorly. Our technique entails circumferential transaction of the aorta both proximally and distally and end-to-end anastomoses to the graft, in contradistinction to other techniques which do not completely transect the aorta. (0 Completed procedure, (g) and (h) These drawings illustrate that less aortic root is resected and replaced when the patient's native aortic valve is preserved (/?) compared to when the valve is replaced (g). In g more of the sinuses of Valsalva have been resected in contrast to h. This difference is also shown in Figures 8-12, 8-14, 8-15, and 8-16.


FIGURE 8-9
Schematic illustration of the mechanism of aortic regurgitation in patients with acute type A dissections. Commissural support of the aortic valve is disrupted by the blood in the false lumen. In the majority of cases the natural aortic valve can be preserved by obliteration of the false lumen and resuspension [see Figures 8-8(d) and (e)]. This diagram also illustrates how the dissection can involve the proximal left main coronary artery without propagating down the artery.
FIGURE 8-12
 D
D iagrammatic
illustration of the operative procedure when preservation of the
patient's aortic valve is not possible. It is important to replace
the aortic valve irrespective of whether itscompetency
can be restored at the time of initial operation in all
patients
with the Marfan syndrome and annuloaortic ectasia. These
have been the only patients in our experience where the long-term
durability of aortic valve resuspension has not been satisfactory.
This inability to guarantee long-term aortic valve competency
is probably related to the marked annular dilatation and attenuation
of aortic valve leaflets in these patients.
iagrammatic
illustration of the operative procedure when preservation of the
patient's aortic valve is not possible. It is important to replace
the aortic valve irrespective of whether itscompetency
can be restored at the time of initial operation in all
patients
with the Marfan syndrome and annuloaortic ectasia. These
have been the only patients in our experience where the long-term
durability of aortic valve resuspension has not been satisfactory.
This inability to guarantee long-term aortic valve competency
is probably related to the marked annular dilatation and attenuation
of aortic valve leaflets in these patients.
As discussed in detail in the text, certain cases of chronic type A aortic dissections may benefit from purposeful redirection of the distal graft flow into both the true and false lumen. This contribution by the UCSF Cardiac Surgical Group entails resection of a small portion of aortic intima. The graft is anastomosed to the false lumen. This technique is applicable to patients with both chronic type A or chronic type B aortic dissections. It must be emphasized that similar concerns and techniques do not apply to patients with acute type A or type B dissections.

FIGURE 8-14
Concomitant aortic valve replacement and ascending aortic replacement. This procedure is rarely necessary in patients with acute type A dissections, but frequently necessary in patients with chronic type A dissections, (a) The conventional technique entails resection of the diseased native aortic valve, separate replacement of the aortic valve (depicted in these illustrations by a porcine bio-prosthesis), and then a separate tube graft replacement for the ascending aorta.

FIGURE
8-14
(d)
This
technique creates small tongues of aortic wall around the left and
right coronary
ostia. If the right (or rarely both) coronary
ostium is displaced markedly cephalad, then such a technique is
inappropriate
(see Figure 8-15). It should be noted
that the suture bites for the proximal
aortic anastomosis are purposefully taken right down at the sewing
ring of the bioprosthetic
aortic valve, except around the coronary ostia. When suturing the
graft around the coronary ostia, it is important to take the
needle bites right into the
ostium of the coronary artery in order to
avoid leaving an undesirable amount of aortic
root remaining, (c) Distal anastomosis
and completed procedure. (From
DC
Miller et al [52].)
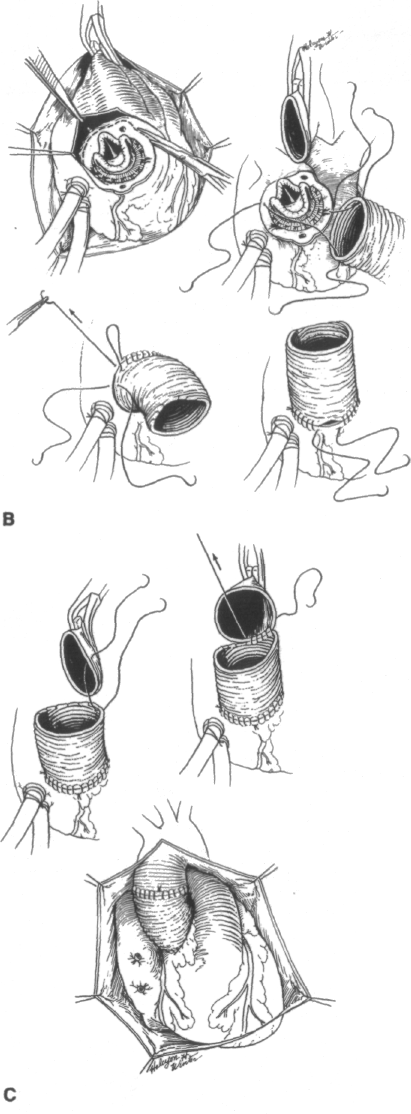
When the coronary ostia are displaced far cephalad, which is frequently the case in the Marfan syndrome and occasionally in patients with annuloaortic ectasia, it is not practical to leave tongues surrounding the coronary ostia without retaining an undue amount of sinus of Valsalva In such cases our technique calls for an end-to-side coronary artery anastomosis to the graft using the Carrel patch technique. This operative technique differs markedly from the "composite technique" where the coronary arteries are anastomosed from within the aneurysm sac "side-to-side." Furthermore, it should be noted that this technique entails resection of the majority of the diseased aorta in contrast to the composite technique where the residual aneurysmal wall is wrapped around the valve-graft conduit for hemostasis. Both aortic anastomoses are end-to-end using the conventional technique, whereas the composite technique entails anastomosing the Dacron graft to the inside of the distal aorta without circumferential full thickness transection of the aorta. From DC Miller et al [52].)


FIGURE 8-19
Operative techniques for surgical repair of type B aortic dissections, (a) Only a conservative segment of the descending aorta is resected, as shown by the dotted lines (see text), (b) Circumferential full-thickness cuffs of aorta are transected and then reconstructed. Intercostal arteries are ligated from within the aortic lumen (arrows), (c) The proximal and the distal anastomoses are then performed sequentially, (d) Completed diagram of the surgical repair of a type B aortic dissection. We do not resect the entire diseased aortic wall, but as shown in the diagram, no attempt is made to preserve the entire aneurysm sac such that it can be wrapped around the aortic graft. Therefore, any anastomotic bleeding points can be controlled under direct vision with additional sutures if necessary.
