
Incidence
Because most previous clinical series have employed the classification of DeBakey et al., this system will be used in the subsequent discussion [4]. Most autopsy series report a higher incidence of proximal as compared to distal dissection in a ratio of approximately 4:1 [5]. It is not unusual, however, for clinical series to include more patients with distal disease [6]. This is because the natural history of the latter is somewhat more prolonged, thus allowing more such patients to arrive at hospital and receive correct diagnosis and therapy.
Men predominate over women by a ratio of approximately 2 or 3 to 1 [7]. Peak incidence of aortic dissection is in the sixth and seventh decades; patients with proximal dissection tend to be younger. Age range is from infancy well into the eighties. Aortic dissection in the younger age groups usually occurs with an asso- ciated predisposing factor, such as congenital heart disease or a connective tissue defect [8]. Rarely no apparent coexisting factors are present on physical examination or by history [9]. The predisposing conditions and pathology of aortic dissection are discussed extensively in Chapter 2.
PRESENTATION
By far, the most common presenting symptom of aortic dissection is pain, which occurs in well over 90 percent of patients. The pain of aortic dissection is rather typical. It is usually of excruciating quality and maximal intensity at its onset, in contrast to the pain of ischemic cardiac disease which may wax and wane. Not uncommonly patients will try to dissipate the pain by moving about, as opposed to patients with angina who more often try to remain motionless. Ironically, patients may describe the sensation as if something "tore" or "ripped" within them. Another characteristic of the pain of aortic dissection is its tendency to migrate to other sites, such as the neck or low back, following the path of the dissecting hematoma as it extends through the aorta. Vasovagal manifestations such as drenching perspiration, apprehension, nausea, vomiting, and faintness are often associated.
The location of pain may be of some help in predicting the site of origin of the dissection [6]. Pain felt maximally in the anterior thorax is more frequent with proximal dissection, whereas pain that is most severe in the interscapular area is much more common with a distal site of origin. Although pain may be felt simultaneously in the anterior and posterior chest with both proximal and distal dissection, the absence of posterior interscapular pain strongly militates against a distal dissection, since over 90 percent of patients with distal dissection report some back pain. Pain in the neck, throat, jaw, or teeth often occurs in dissections that involve the ascending aorta or arch (see Table 4-1).
Other less common, but recognized presenting symptoms of acute aortic dissection include syncope, stroke, paraplegia, pulse loss with or without ischemic pain, and congestive heart failure with or without associated chest pain. Aortic dissection can be asymptomatic although a majority of patients without pain have suffered some disturbance of consciousness as a result of their dissection [10].
In a clinical series from the Massachusetts General Hospital published by Slater and DeSanctis, 94 percent of 124 patients presented with pain as a primary symptom [6]. Of these, six patients experienced syncope in addition to pain, five presented with associated congestive heart failure, and two with an associated stroke. Eight patients presented pain-free: four with congestive heart failure, two with cerebrovascular accident and altered consciousness, and two with an abnormal chest roentgenogram who recalled no prior episode of pain.
The presence of syncope may bear a special connotation in aortic dissection since five of the above six patients who experienced syncope were subsequently found to have extension of their dissection into the pericardial cavity with tam-ponade. Congestive heart failure almost always derives from associated aortic
valve insufficiency, the signs of which may be masked by the elevated filling pressure.
Alternatively, those patients who present with chronic aortic dissection comprise a subset of individuals who often recall no prior episode of pain or an antecedent event with pain which was incorrectly diagnosed. These patients generally come to clinical awareness because of aortic insufficiency or associated congestive heart failure.
PHYSICAL EXAMINATION
The manifestations of aortic dissection in any given patient are determined by the path taken by the dissecting hematoma as it progresses through the aorta. Thus, the circulation of any major artery arising from the aorta may be compromised; disruption of the support of the aortic valve by extension into the aortic root may cause aortic incompetence; and finally the dissecting column may rupture through the adventitia anywhere along the aorta, although the two commonest sites of rupture are the pericardial space and the left pleural cavity [11). It follows that the primary location of the dissection, whether it be proximal or distal, will do much to determine the major manifestations of this disease. Thus, the expected physical findings in patients with aortic dissection depend largely
upon whether ascending or descending aorta is involved by the dissecting hema-toma. Having taken the above into account, one can often make the diagnosis of aortic dissection with reasonable assurance based upon the physical examination alone.
As emphasized by Lindsay and Hurst, patients with aortic dissection may often present with markedly elevated blood pressure, even though they may appear to be in shock; this is especially true for patients with distal dissection [12]. In their series, 42 percent of patients with distal disease had a diastolic pressure of 140 mmHg or greater on admission, and over 60 percent had diastolic pressure elevations of 120 mmHg or more. Many of these patients have severe preexisting hypertension which is presumably aggravated by the catecholamine response to severe pain. Conversely, hypotension (systolic blood pressure below 100 mmHg) was found in 20 percent of patients with proximal dissection and none of the patients with distal dissection. Hypotension can result from hypovolemia due to hemorrhage into the pleural or peritoneal space, pericardial tamponade, or heart failure due to aortic insufficiency. Alternatively, dissection into the brachial artery with direct compression of the arterial lumen or obliteration of the subclavian artery orifice by the intimal flap, may produce pseudohypotension, the inability to detect blood pressure by the standard cuff method in one or both arms despite true elevation of blood pressure.
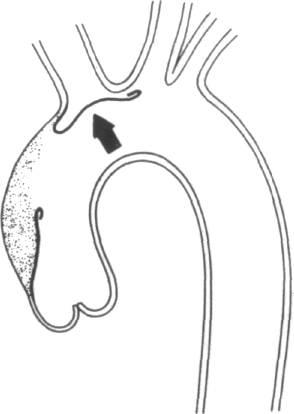
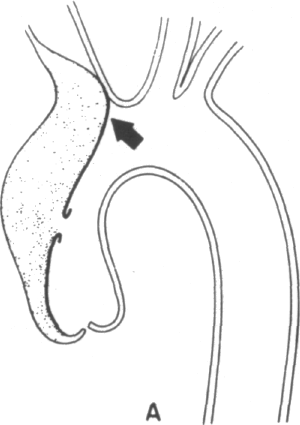
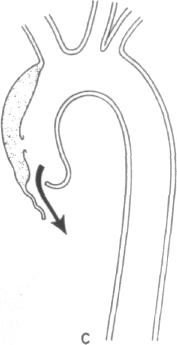
Clearly, certain predisposing factors will be appreciated on physical examination: the stigmata of Marfan's, Ehlers-Danlos, or Turner's syndromes may be apparent. The most commonly associated physical findings in aortic dissection include pulse loss, aortic insufficiency, and neurological deficit. Examination of the peripheral pulses are requisite to evaluate patients in whom the diagnosis of aortic dissection is being entertained. The finding of a difference in arm blood pressures or of an absent or diminished arterial pulse serves as a key diagnostic clue. The mechanisms of pulse loss are depicted in Figure 4-1. In addition to these mechanisms, the rare occurrence of intimo-intimal intussusception with resultant pulse loss has been reported [13]. Pulse loss may be transitory due to local reentry of the hematoma into the artery itself or, alternatively, due to movement of the intimal flap away from the orifice. Occasionally, reduplication of pulses may be appreciated by palpation, the reduplication being caused by nonsimultaneous blood flow in true and false lumens.
Clearly, the primary location of the dissecting hematoma will determine the locations and frequency of pulse loss. In the clinical series of Slater and DeSanctis, almost half of the patients with proximal disease and 16 percent of patients with distal disease evinced the loss of one or more pulses [6]. In the former patients, deficits were found evenly distributed along the entire arterial tree, from right carotid to the femorals; in the latter group, deficits included left subclavian and femoral vessels, except in one patient who experienced retrograde extension of her hematoma into the aortic arch. Clearly if congenital abnormalities of the aortic arch are present, then predictions of pulses in jeopardy from proximal or distal dissection will no longer hold [14].
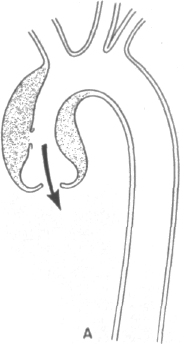
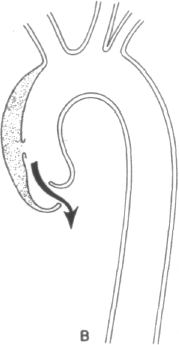
The mechanisms of aortic insufficiency are outlined in Figure 4-2 and may derive from (1) a circumferential hematoma widening the aortic root and separ-
ating the aortic cusps, (2) displacement of one cusp substantially below the level of the others by asymmetric pressure of the dissecting hematoma, or (3) actual disruption of the annular leaflet support leading to a flail cusp. These mechanisms bear upon ultimate therapy. In the former two instances, simple surgical decompression of the hematoma should allow full restoration of valve competence. In the latter case, however, an aortic valve prosthesis would be required.
Clearly, ascending aortic involvement is required to produce dissection-related aortic insufficiency. In most clinical series one-half to two-thirds of patients with proximal dissection develop the murmur of aortic insufficiency [6,12]. Conversely, fewer than 10 percent of patients with distal disease have aortic insufficiency, and in the majority of these cases, the murmur derived from a condition which antedated the dissection, namely severe hypertension, valvular heart disease, or annu-loaortic ectasia. Rarely, distal dissection advances retrograde to involve the aortic root.
The murmur of aortic insufficiency often has a musical quality and may radiate to the right rather than to the left sternal border. The murmur may be of variable intensity, depending upon the height of arterial blood pressure. Depending upon the severity of the insufficiency, other peripheral signs of high output such as widened pulse pressure and bounding pulses may coexist. Conversely, in cases where heart failure dominates the clinical picture, it may mask the aortic murmur. Rarely, the presence of disproportionately bounding pulses in the face of severe heart failure may serve as a clue to the diagnosis of aortic dissection. Heart failure in a patient with aortic dissection almost always indicates aortic valve insufficiency.
Patients who present with chronic aortic dissection generally have acquired all the well-known stigmata of chronic aortic insufficiency: widened pulse pressure and its associated signs, a dicrotic notch on carotid palpation, normal heart rate unless congestive failure has supervened, cardiomegaly, and a prolonged regurgi-tant murmur.
The signs of pericardia! effusion or tamponade—for example, hypotension, pulsus paradoxus, or a friction rub—should be searched for in patients with aortic root involvement While hemorrhage into the pericardial sac is the most frequent cause of death in aortic dissection, clinical series generally record an incidence of clinically apparent pericardial effusion of between 5 and 10 percent [5,6,11]. Lastly, signs of coexisting predisposing cardiac anomalies such as aortic co-arctation, bicuspid aortic valve, or aortic valve stenosis may be present on cardiac examination.
The neurological deficits of aortic dissection, which have been reviewed by Weisman and Adams, include cerebrovascular accident, ischemic paraparesis, and ischemic neuropathy [15]. In general, hemiparesis with or without disturbances of consciousness occurs in patients with proximal disease, and neurological compromise of the lower extremities is seen in patients with distal disease. Disturbances of consciousness may derive directly from vascular accident or indirectly from hy-poperfusion as a result of hypotension from its several causes. Neurological deficits result from obstruction of the blood supply to cerebral hemispheres, extremities, or
spinal cord by the hematoma itself or in the intimal flap. These deficits occur in fewer than half of patients with aortic dissection and predominate in those patients with proximal disease. Frequency in most series is approximately 20 percent [5].
The onset of monoplegia is typically abrupt with symptoms of pain, dysesthesia, or hypesthesia and is followed by anesthesia. Examination reveals the limb to be cold, pulseless, mottled, and paralyzed with absent deep tendon reflexes. Clearly, aortic dissection must be considered in the differential diagnosis of an ischemic extremity. Dissection of intercostal or lumbar arteries, in particular, interruption of flow to the artery of Adamkiewicz which provides primary blood supply to the spinal cord, can result in cord ischemia which manifests itself as flaccid or spastic paraplegia, and anesthesia for pain and temperature below the level of the cord involvement [16]. The Babinski reflex is generally present and bilateral. Position sense is generally maintained. Sphincter tone is usually affected.
Strokes, when they occur, are clinically indistinguishable from interruption of cerebral blood supply from any cause, such as thrombosis or embolus. Neurological compromise from aortic dissection is rarely reversible and is associated with a generally poor prognosis.
A host of additional physical findings has been associated with aortic dissection, and each derives from interruption of blood supply, compression by the hematoma, or extension of the hematoma. These include Homer's syndrome due to compression of superior cervical ganglion, a pulsatile sternoclavicular joint [17], vocal cord paralysis and hoarseness due to pressure against the left recurrent laryngeal nerve, superior mediastinal syndrome from superior vena cava obstruction [18,19], pulsating neck masses, tracheal or bronchial compression, hemoptysis due to erosion into the bronchus by hematoma [20], respiratory distress or bron-chospasm due to bronchial obstruction by the hematoma, hematemesis due to penetration into the esophagus [21], hemorrhagic pleural effusion which almost invariably occurs on the left side, myocardial infarction, heart block due to dissection down the interatrial septum into the AV node [22,23], a continuous murmur due to rupture into the right atrium or ventricle [24-26J, symptoms and signs of mesen-teric or renal infarction, and fever [28]. Although high fever is uncommon, it can occur presumably as a result of the release of pyrogenic substances from the hematoma or associated pleural effusions. It can persist for a protracted period of time and is often misdiagnosed as endocarditis or mycotic aneurysm. Pleural effusion occurs in approximately 10 percent of patients. The clinical incidence of myocardial, renal, or mesenteric infarction is estimated at between 1 and 2 percent. The other complications occur much more rarely.
LABORATORY STUDIES
Routine laboratory studies are not of great benefit in making the diagnosis of aortic dissection, except to rule out alternative diagnostic possibilities. Occasional patients will be anemic as a result of dissection-related sequestration of blood. A mild leukocytosis (10,000 to 14,000 leukocytes per cubic millimeter) can occur in
acute dissection, and is most probably stress-related. Rarely, disseminated intra-vascular coagulation has been observed [28]. This is manifested by a prolonged prothrombin time and elevation of fibrin-split products. Lactic acid dehydrogenase (LDH) and bilirubin levels are sometimes elevated due to hemolysis of blood within the false lumen. Values for the cardiac enzymes serum glutamic oxaloacetic transaminase (SGOT), creatine phosphokinase (CPK), and the CPK-MB fraction are usually normal. The electrocardiogram is useful to rule out active cardiac ischemia and more commonly will reveal evidence of prior hypertension (left atrial enlargement or left ventricular hypertrophy). Of course, in the rare patient with dissection-related myocardial infarction, active ischemia will be noted. Occasionally, low voltage or electrical alternans as a result of pericardial effusion will be observed.
While the roentgenographic features of aortic dissection will be discussed in detail in Chapter 5, their use in diagnosis deserves mention. Provided that a proper upright posteroanterior film is taken, evidence for an aortic dissection should be discernible in roughly 85 percent of cases [6,29]. Alternatively, it is possible for extensive aortic dissection to occur without radiographic abnormalities, in particular if the hematoma occupies the vascular lumen without widening the aortic silhouette [30]. Comparison with previous films often enhances diagnostic capability.
Currently, the definitive diagnosis and delineation of aortic dissection is made on the basis of angiography. The angiographic features of dissection are depicted and detailed in Chapter 5. Moreover, certain adjunctive diagnostic procedures, in particular, echocardiography, CT scanning, and digital angiography bear mention, and there is a possibility that the latter two techniques eventually may prove useful in primary diagnosis.
Because of the time required to properly perform both M-mode and two-dimensional echo studies, their less reliable diagnostic potential, and their use in proximal dissections only, echocardiography is currently favored as an ideal noninvasive method of following up patients with treated ascending aortic dissection rather than to make the primary diagnosis [31].
On the other hand, preliminary experience with CT and digital angiography at our institution and elsewhere suggests that they may provide prompt and valuable information about aortic size while obviating the small but significant risks of arteriography [32,33]. Thus as experience accumulates, we expect successful application of these techniques in both the primary diagnosis and follow-up of patients with aortic dissection. These noninvasive diagnostic techniques are discussed in detail in Chapters 5, 6, and 8.
In summary, although the diagnosis of aortic dissection is often readily apparent from the constellation of symptoms and signs discussed above, it can present in many subtle guises. Moreover, the relative rarity of aortic dissection in contrast to ischemic cardiac disease serves to desensitize the admitting physician. It is hoped that this chapter will serve to remind clinicians of the protean manifestations of this devastating disease.
SURGICAL MANAGEMENT
OF AORTIC DISSECTIONS:
