
Incidence
Aortic dissection is an uncommon, but not rare, disease. Large academic referral centers with a long-standing interest in aortic dissection see from 8 to 12 cases per year [50,51].
The incidence of aortic dissection varies in different population groups and is probably most closely related to the frequency of hypertension. Anagnostopoulos estimated that approximately 2000 cases of acute aortic dissection occur in the United States each year [52]. In Denmark, the estimated incidence of aortic dissection was placed at 5.2 per million per year [53].
The autopsy incidence of aortic dissection is somewhere between 1 in 350 and 1 in 500. Hirst et al. reviewed 31 series from the world's literature prior to 1958 and found 482 dissections in 175,405 patients, or 1 in every 363 autopsies [9]. In another series 1 aortic dissection was encountered in every 498 autopsies [53]. In Denmark, aortic dissection was found to be the most common catastrophic event involving the aorta, being 50 percent more common than a ruptured abdominal aneurysm and more than 4 times as common as a ruptured thoracic aneurysm [53].
CLASSIFICATION
A knowledge of the classification of aortic dissection is essential for a proper understanding and management of the disease. Classification is based upon duration and anatomic location of the dissection. These variables are also the two main determinants of both prognosis and choice of therapeutic modality.
Duration of Dissection: Acute vs. Chronic
Acute dissection is defined as having occurred less than 2 weeks prior to the institution of therapy. Chronic dissection is defined as having occurred more than 2 weeks prior to the institution of therapy. This definition, while arbitrary, emphasizes the better prognosis for patients who survive beyond the acute phase of dissection [40,48,50,51,54,55]. The further subdivision of aortic dissections into acute, subacute, and chronic is unnecessary [5,46,52,56,57].
Anatomic Location
Most classifications of aortic dissection are based upon the site of the intimal tear and the extent of the dissecting hematoma.
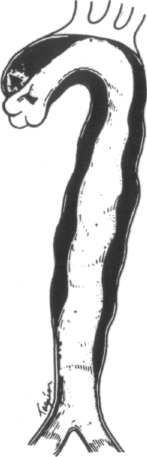
The most widely used classification is that of DeBakey et al. (Figure 1-2) [40]. They define three types of dissection based upon the site of intimal tear and extent of the dissecting hematoma. In type I and type II dissection, the intimal tear is located in the ascending aorta, usually just a few centimeters above the aortic valve. In type I dissection, the hematoma extends for a variable distance beyond the ascending aorta, whereas in type II dissection the hematoma is confined to the ascending aorta. In type HI dissection, the hematoma originates in the descending aorta, usually just beyond the origin of the left subclavian artery. The dissection usually propagates antegrade into the descending aorta, or rarely retrograde into the aortic arch and ascending aorta. Occasionally, the intimal tear is present in an unusual location, such as the aortic arch [9,34,42,47,48,58,59] or the abdominal aorta [9,34,42,60-62]. In approximately 4 to 5 percent of cases, no intimal tear can be found [9]. In these situations, the dissection is classified according to the extent of the dissecting hematoma.
Since type I and type II dissections often behave similarly, most investigators refer to them as proximal, or ascending, dissections. Type III lesions are often termed distal, or descending, dissections.
Recently, Daily et al. [63] and Miller et al. [50] at Stanford have proposed a simpler classification based only upon the presence or absence of involvement of the ascending aorta. In their classification, type A refers to all dissections that involve the ascending aorta. The arch or distal aorta may or may not be involved and the intimal tear may be located anywhere along the course of the aorta. All other dissections are classified as type B. In these patients the dissection is usually confined to the aorta distal to the left subclavian artery.
Since involvement of the ascending aorta is an important determinant of both prognosis and choice of therapeutic modality, we prefer the classification of aortic dissections as proximal or type A (ascending aorta involved) and distal or type B (ascending aorta not involved).
Several other methods of classification of aortic dissection have been proposed. Shennan provided a detailed pathological description of the location and extent of dissection in 287 of 300 cases [34]. He also described the features and extent of the intimal tear but did not formulate a specific classification based on these features. Kirkpatrick [64] and Hume and Porter [42] divided dissections into six types based upon the site of intimal tear and extent of the dissecting hematoma. Reul et al. further subdivided DeBakey type III dissections into subgroups a-d based upon the extent of the dissecting hematoma [48]. Anagnostopoulos [52] and Kolff et al. [59] proposed a method of classification based upon the anatomy of the dissection, the presenting complications, the presence of opacification of the false lumen on angiography and the preferred method of therapeutic management. None of these proposed methods of classification has gained wide acceptance.
 Rather
than insist on uniformity throughout this monograph, we have allowed
the
individual authors to employ whichever classification scheme they
prefer.
Rather
than insist on uniformity throughout this monograph, we have allowed
the
individual authors to employ whichever classification scheme they
prefer.
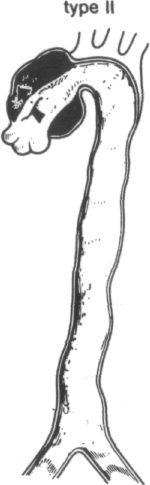
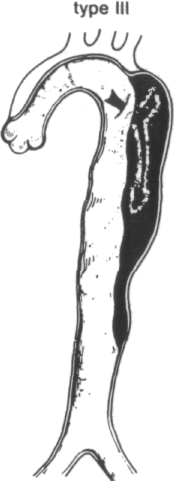
FIGURE 1-2
Systems for classifying aortic dissections. In the most widely used classification system (that of DeBakey) types I and II originate with an intimal tear (single arrows) in the ascending aorta near the aortic valve, whereas type III dissections originate in the descending thoracic aorta (double arrow). Type I lesions extend for variable distances beyond the ascending aorta, type II lesions are confined to the ascending aorta, and type III lesions may propagate distally or, more rarely, proximally into the arch and ascending aorta. Types I and II are now commonly called proximal, or ascending, dissections, and type III, distal, or descending, dissections. A recent simpler classification disregards the site of origin and calls all dissections that involve the ascending aorta type A and those that do not, type B. (From R Doroghazi, EE Slater, RW DeSanctis: Medical therapy for aortic dissections. J Cardiovasc Med 6:187, 1981.)
THE ETIOLOGY AND
PATHOLOGY OF AORTIC
DISSECTION
Albert E. Hirst Ira Gore
HISTOLOGY OF THE HUMAN AORTA
Structurally the aorta is composed of three layers. The inner, or intimal, layer consists of a thin layer of collagenous tissue containing myofibroblasts, elastic fibers, and smooth muscle cells lined by a continuous sheet of endothelial cells. The innermost layer of elastic tissue of the media serves to separate the intima from the media since there is no distinctive internal elastic lamina [1].
The media, the chief supporting layer of the aorta, lies just exterior to the intima and is composed of concentrically arranged fenestrated layers of lamellae of elastic tissue interconnected by a delicate network of elastin fibrils (Figure 2-1). Between the elastic laminae, collagen fibers and smooth muscle cells are embedded in a matrix of ground substance. The ground substances of the aorta are connective tissue proteoglycans in which a number of polysaccharide chains are covalently linked to a protein core. The designations of the predominant acid mucopolysaccharides have undergone recent revision to include the following categories: chondroitin 4-sulfate (formerly chondroitin sulfate A), chondroitin 6-sulfate (formerly chondroitin sulfate C), dermatan sulfate (chondroitin sulfate B), and heparan sulfate (formerly heparitin sulfate and heparan monosulfate) [2]. Of these proteoglycans, only dermatan sulfate has been found to have anticoagulant properties. [3].
The smooth muscle cell is the only cell encountered in the media and appears to be the precursor of the other structural elements of the media. Synthesis of elastic tissue and collagen by smooth muscle cells of the aorta has been demonstrated in the pig and monkey by Ross [4]. Although collagen is found between the elastic laminae, fibroblasts have not been identified despite search using electron mi croscopy [5]. It has been suggested [6] that the muscle fibers normally insert into the elastic laminae to serve in an auxiliary fashion to limit the expansion of the elastic tissue. Some electron microscopic support for this concept was reported by Moss and Benditt [7] who found apparent fusion of the smooth muscle basement membrane with the hazy mantle surrounding the elastic fibers in the aorta of chickens.
The adventitia is a structurally tough layer composed of irregularly arranged collagenous tissue incorporating circumferentially oriented elastic fibers. Within the adventitia are small vessels, the vasa vasorum, which usually arise from branches of the aorta and ramify in the adventitia of the aorta before penetrating the outer third of the media to arborize in a plane between the middle and outer third of the media [8]. Atherosclerosis may greatly alter the distribution of the vasa vasorum, resulting in their extension into the intima and even their origin from the aortic lumen [9].
PHYSIOLOGICAL CONSIDERATIONS
The proximal segment of the aorta is subjected to the greatest strain of any portion of the aorta since it must withstand the full unbuffered force of ventricular systole and diastole. The wall of the ascending aorta is also under greater tension than more distal segments because of its greater diameter. As the Laplace principle indicates, the circumferential tension varies with the luminal diameter as well as with the intravascular pressure. Thus, the tension on the wall of the vessel is directly proportional to its radius if the luminal pressure is unchanged. The lesser diameter of the abdominal aorta, 30 to 40 percent smaller than that of the thoracic segment [10], permits its thinner wall to withstand intraluminal tension.
Between birth and maturity the thickness of both the thoracic and the abdominal aorta more than doubles. In the thoracic segment, growth is largely the result of an increase in lamellar units, a lamellar unit being defined as an elastin lamella and the contents of its adjacent interlamellar zone [11]. In contrast, maturation in the abdominal segment results principally from thickening of the individual units with only a slight increase (from 25 to 28) in their number [12].
To accommodate the Laplace effect, the proximal aorta is thicker and contains a greater proportion of elastic tissue than the distal segments. An average of 56 lamellar units is found in the proximal segment but only 28 lamellar units in the distal segment [12].
The association of elastic tissue and collagen in the aorta permit it to function as a "two-phase" material in which distensibility and pulsation within and close to the physiological range is supported chiefly by the elastic tissue; at higher pressures approaching or exceeding the limits of elastic distensibility, circumferentially oriented collagen fibers assume the strain [13].
In 1928, Gsell [14] described the histological changes he encountered in eight cases of aortic dissection. Focal areas of necrosis principally involving muscle were the initial lesion and were followed by degeneration of elastic tissue and collagen to produce clefts, sometimes containing mucoid ground substance. The repair tural study of the aortic lesions in one case [21] revealed that the cellular and extracellular components were morphologically normal but haphazard in arrangement and relative proportions. The collagen formed by fibroblasts cultured from patients with Marfan's syndrome is more soluble than that from controls and favors the hypothesis that a molecular defect interferes with the formation or stabilization of crosslinks between its subunits of collagen fibers [22,23].
Typically, elastic tissue lesions consist of variable sized faults in the media in which the elastic lamellae have become depleted and fragmented, and the resulting defects become filled with semifluid ground substance. Secondarily there is a loss of alignment of muscle cells which have lost the scaffold influence of adjacent elastic laminae, strongly suggesting the interdependence of muscle cells and elastic laminae (Figure 2-2). Elastic lesions are thus most devastating to the media since they result in a loss of integrity of both major supporting elements of the vessel wall, i.e., elastic tissue and muscle. In such cases aortic dissection often develops in the absence of preexisting hypertension. It seems reasonable to propose that the term cystic medionecrosis has outlived its usefulness and should be replaced by the more precise designation, elastic tissue lesion as we have previously recommended [24]. It does not seem justifiable to apply the term cystic medionecrosis to the aortic media, which shows only a slight increase or vacuoli-zation of the ground substance in the absence of other structural alterations. About one-third of patients with arachnodactyly who have come to autopsy have reaction was scanty and resulted in an imperfect scar. He named the lesion me-dionecrosis idiopathica. Subsequently Erdheim, in 1929 and 1930 [15,16], described in meticulous detail the mucoid and cystic lesions which he encountered in two cases, not of aortic dissection, but of spontaneous rupture of the ascending aorta. He used the term medionecrosis aortae idiopathica in his first article and appended the term cystica in the second. He emphasized that the accumulations of mucoid substance at first lacunar in size gradually coalesced to form cysts which interrupted the continuity of elastic tissue and collagen, although the muscle cells occasionally persisted. The mucoid substance stained metachromatically. His second article emphasized that the mucoid changes which began initially in the interlamellar spaces were associated with disappearance of the muscle cells, collagen, and fine elastic fibers. The lesions were essentially noninflammatory and were not related to changes in the intima, adventitia, or vasa vasorum. He stressed the lack of a healing reaction and the resultant impairment of strength which predisposed to aneurysmal dilatation and rupture.
In the intervening years, Erdheim's lesion has been generally accepted as the cause-of the majority of cases of aortic dissection. However, the fact that Erdheim's lesion has been reported to occur over a wide range from as low as 0 percent to as high as 83 percent of cases [17] indicates that a wide difference of opinion exists regarding the criteria which should be considered diagnostic of the lesion. Some have considered as diagnostic a slight increase in ground substance, particularly when it is foamy or vacuolated, in the absence of structural alterations in either the elastic tissue or smooth muscle of the media. Controlled studies, such as those by Hurley [18] and Manley [19] have revealed that these features are common in many otherwise normal aortas. While Erdheim apparently regarded the accumulation of ground substance as the primary event and the destruction of elastic tissue, muscle, and collagen as secondary, the reverse of this pathogenesis is generally favored today; semifluid ground substance increases in response to medial injury and, under the influence of intramural tension, accumulates in voids left by focal destruction of medial constituents.
In order to classify the histological lesions encountered in aortic dissection, it seems advisable to describe the changes resulting in the media when one or more of its chief supporting elements are missing.
DEFECTS IN ELASTIC TISSUE
Elastic tissue lesions were found to predominate in aortic dissection in people below the age of 40 [6]. Similarly, lesions in the aorta associated with aortic dissection in arachnodactyly (Marfan's syndrome) have been consistently of the type which Erdheim described. The evidence strongly suggests that elastic tissue defects are the underlying cause for aortic dissection in these patients. The exact site of defective organization and maturation of collagen in the autosomal dominant form of the disease, the most common type, has not been determined. Earlier studies by Sjoerdsma in 1958 [20] in which hydroxyproline excretion was increased in the urine suggested an increase in collagen metabolism. Ultrastruc- croscopy (5). It has been suggested [6] that the muscle fibers normally insert into the elastic laminae to serve in an auxiliary fashion to limit the expansion of the elastic tissue. Some electron microscopic support for this concept was reported by Moss and Benditt [7] who found apparent fusion of the smooth muscle basement membrane with the hazy mantle surrounding the elastic fibers in the aorta of chickens.
The adventitia is a structurally tough layer composed of irregularly arranged collagenous tissue incorporating circumferentially oriented elastic fibers. Within the adventitia are small vessels, the vasa vasorum, which usually arise from branches of the aorta and ramify in the adventitia of the aorta before penetrating the outer third of the media to arborize in a plane between the middle and outer third of the media [8]. Atherosclerosis may greatly alter the distribution of the vasa vasorum, resulting in their extension into the intima and even their origin from the aortic lumen [9].
PHYSIOLOGICAL CONSIDERATIONS
The proximal segment of the aorta is subjected to the greatest strain of any portion of the aorta since it must withstand the full unbuffered force of ventricular systole and diastole. The wall of the ascending aorta is also under greater tension than more distal segments because of its greater diameter. As the Laplace principle indicates, the circumferential tension varies with the luminal diameter as well as with the intravascular pressure. Thus, the tension on the wall of the vessel is directly proportional to its radius if the luminal pressure is unchanged. The lesser diameter of the abdominal aorta, 30 to 40 percent smaller than that of the thoracic segment [10], permits its thinner wall to withstand intraluminal tension.
Between birth and maturity the thickness of both the thoracic and the abdominal aorta more than doubles. In the thoracic segment, growth is largely the result of an increase in lamellar units, a lamellar unit being defined as an elastin lamella and the contents of its adjacent interlamellar zone [11]. In contrast, maturation in the abdominal segment results principally from thickening of the individual units with only a slight increase (from 25 to 28) in their number [12].
To accommodate the Laplace effect, the proximal aorta is thicker and contains a greater proportion of elastic tissue than the distal segments. An average of 56 lamellar units is found in the proximal segment but only 28 lamellar units in the distal segment [12].
The association of elastic tissue and collagen in the aorta permit it to function as a "two-phase" material in which distensibility and pulsation within and close to the physiological range is supported chiefly by the elastic tissue; at higher pressures approaching or exceeding the limits of elastic distensibility, circumferentially oriented collagen fibers assume the strain [13].
In 1928, Gsell [14] described the histological changes he encountered in eight cases of aortic dissection. Focal areas of necrosis principally involving muscle were the initial lesion and were followed by degeneration of elastic tissue and collagen to produce clefts, sometimes containing mucoid ground substance. The repair
had aortic dissection as a cause of death, and the average age at death has been 32 years [25]. Lesions of the elastic tissue type have also been responsible for aneu-rysms limited to the ascending aorta occurring in the absence of positive serolog-ical tests for syphilis or the skeletal features of Marfan's syndrome [26].
LESIONS OF MUSCLE
Smooth muscle cells, with their ability to contract, play an active role in support of the media. Since they are viable nucleated cells, they are highly sensitive to ischemia. In Gore's review of aortic dissection [6], muscle lesions predominated after the age of 40. The resultant lesions are characterized by the formation of an acellular zone in which muscle cells have disappeared (Figure 2-3a). The elastic laminae which persisted in such zones were condensed, closer together, and had lost their wavy pattern as though stretched (Figure 2-36). The latter effect may be the result of a loss of the moderating effect of the muscle cells on the elongation of the elastic laminae. The lesions often involve the midmedia which is most distant from both sources of nutrition (i.e., the intima and adventitia). Because the lesions are often limited to a particular stratum in the media, they have been called zones of laminar loss of nuclei or laminar necrosis. Since muscle cell lesions do not destroy the integrity of the elastica, they probably produce less impairment of tensile strength of the aorta than do elastic lesions. For reasons as yet not clear, muscle lesions do not tend to accumulate ground substance as do elastic lesions. The extent to which muscle lesions impair the resistance of the aorta to intramural dissection is not currently known but the fact that dissections not infrequently occur through a zone of muscle necrosis would suggest that they may play a part in initiating aortic dissection. Barsky and Rosen [27] used the term aortic infarction to describe such lesions, which they believed to follow aortic dissection, since they did not find similar lesions in age-matched controls. This has not been the experience of others (see The Specificity of Medial Lesions, below).
COMBINED MEDIAL LESIONS
Some cases of medial degeneration include both elastic and muscle lesions and the designation idiopathic medial aortopathy and arteriopathy has been proposed by Marquis et al. [28]. The authors describe the gross changes of a white cobblestone thickening of the intima, not unlike the changes seen in syphilitic aortitis, and suspect that the lesions may represent an autoimmune process. The lesions occurred in the absence of syphilis and often in association with ectasia or an-eurysm of the aorta. Clinical conditions in which the lesion was said to occur included a variety of diseases such as ankylosing spondylitis, scleroderma, Rei-ter's syndrome, rheumatoid arthritis, aortic arch arteritis, giant cell arteritis. and granulomatous aortitis. Only the latter two have been found to predispose to aortic dissection and are discussed in more detail under Giant Cell Aortitis, below.
CAUSES OF AORTIC DISSECTION
Pregnancy
A remarkable predisposition to the occurrence of aortic dissection in pregnancy has been known for many years. In 1944, Schnitker and Bayer [29] were able to collect 49 instances of aortic dissection in females below the age of 40; 20, or 49 percent, occurred in association with pregnancy. Ten years later, Mandel et al. [30] found a total of 70 cases of aortic dissection under the age of 40; 36, or 50 percent, were associated with pregnancy. Hirst et al. [17] encountered 15 instances of aortic dissection in pregnancy in a collected study of 505 cases of dissection reported from 1934 to 1954. Aortic dissection was associated with pregnancy in 12 of 25, or 48 percent, of females below the age of 40. A comprehensive survey of aneurysms by Pedowitz and Perell in 1957 [31] revealed a variety of aneurysms in pregnancy including 48 cases of aortic dissection; 29 fusiform or saccular aneurysms of the aorta; and 36 splenic, 10 renal, and 5 iliac aneurysms. Dissections were uncommon (only 2 percent) in the first trimester, but increased to 17 percent in the second trimester, reaching a peak of 48 percent in the third trimester. Only 12.5 percent occurred during labor or within 24 hours thereafter, while 21 percent occurred later in the postpartum period.
Factors suspected to play a part in the occurrence of aortic dissection in pregnancy may be grouped into three categories including circulatory changes and the stress and strain of labor, hormonal changes, and presence of associated diseases (Marfan's syndrome, coarctation, and hypertension).
Circulatory Changes The basal blood pressure, especially diastolic, is lower during pregnancy. The pulse rate is increased. There is also an increase in cardiac output by as much as 50 percent during pregnancy. After delivery the blood volume is usually within normal limits [32]. Such changes do not account for the tendency of aortic dissection to occur during the last trimester of pregnancy and the puerperium. Similarly, the period of greatest stress and strain in pregnancy, i.e., during delivery, is not associated with a high incidence of dissection (i.e., only 12.5 percent).
Hormonal Changes Certain hormones are greatly increased during pregnancy including gonadotropins, progesterone, and estrogen. Of particular interest is the hormone relaxin, which is known to produce softening of the symphysis pubis in the guinea pig and other small animals, although its biological effect in humans is not clear [33].
Danforth et al. [34] attempted to relate changes in the media of the aorta in rabbits to hormonal influences. Consistent findings in the aortas of adult pregnant female rabbits included increase in smooth muscle, fragmentation of reticulum, attenuation of elastica, and reduction in ground substance when compared to controls. Enovid (norethynodrel with mestranol), given in doses sufficient to suppress ovulation, was found to have similar effects.
Johnson et al. [35], using a similarly designed experiment in rabbits, were unable to demonstrate physical differences in the elasticity of the aorta (i.e., length-tension
characteristics) which would be expected if pregnancy produced degenerative changes in the aortic media.
In 1967, Manalo-Estrella and Barker [36] reported that histological alterations occurred in aortas of pregnant women similar to those previously described in the rabbit. Two years later Cavanzo and Taylor [37] were unable to find histological differences in the aorta between 43 pregnant women and 20 controls in any of the 3 trimesters of pregnancy.
Marfan's syndrome, coarctation, and hypertension The association between Marfan's syndrome (arachnodactyly) and aortic dissection has been recognized only in recent years, the first case in the English literature having been reported in 1943 [38]. The association of aortic dissection with arachnodactyly was not mentioned in Pedowitz and Perell's review article in 1957 [31]. Hirst et al. in 1958 [17] found one and possibly two of fifteen dissections in pregnancy associated with Marfan's syndrome. Seven of thirteen recently reported cases of aortic dissection in pregnancy, not included in Pedowitz and Perell's study, revealed arachnodactyly (Table 2-1).
Anomalies of the aortic arch, including 8(17 percent) with coarctation and 2 (4 percent) with aortic hypoplasia were encountered in the 48 dissections in pregnancy collected by Pedowitz and Perell [31], a combined incidence of 21 percent. Coarctation was present in 3 of 15 (20 percent) of cases in pregnancy reviewed by Hirst et al. [17]. Two of thirteen recently reported cases had coarctation of the aorta (Table 2-1).
Mendelson [49] found that coarctation of the aorta exerted an unfavorable effect on pregnancy. Among 26 cases collected from the literature prior to 1940, 5 (19 percent) died and 8 (30 percent) became worse during pregnancy. However, Deal and Wooley [50] reported on a series of 83 recently reported patients including 28 of their own collected between 1956 and 1970 in which there was no maternal mortality or morbidity, suggesting that the effect of coarctation on pregnancy is not as unfavorable as has been reported previously.
Hypertension has been a frequent finding in dissections in pregnancy. While only 2 of 8 (25 percent) of cases of Schnitker and Bayer [29] had hypertension, 19 of 40 (48 percent) of Pedowitz and Perell's cases [31] and 5 of 10 cases of Hirst et al. [17] were hypertensive. As Table 2-1 indicates, elevated arterial pressure was a factor in only 3 of 13, or 23 percent, of the cases of dissections in pregnancy reported since 1956.
While there is more to be learned about the uncommon association of aortic dissection with pregnancy, the available information indicates that Marfan's syndrome, hypertension, and coarctation contribute significantly to the genesis of aortic dissections in pregnancy just as they do in the nonpregnant female.
Aortic Valve Stenosis
The Obscure Mechanism of Poststenotic Dilatation Stenosis of the aortic valve and coarctation of the aorta may result in the phenomenon known as
poststenotic dilatation in which a localized enlargement occurs immediately distal to a site of constriction in an artery. While this phenomenon has been known for many years, the mechanism remained obscure until clarified by the experiments of Holman in 1954 [51]. Using a pump capable of duplicating arterial pulsatile pressure changes in a segment of rubber tubing, permanent dilatation of the tubing developed beyond a site of severe constriction after a period of many (31 to 90) hours. The narrow column of fluid passing through the constricted segment under high velocity struck the more slowly moving fluid mass just beyond the constriction, resulting in turbulence and increased lateral pressure. With time, permanent dilatation of the poststenotic segment resulted from structural fatigue. No doubt the increased tension on the wall resulting from dilatation contributed to aneurysm formation. Although Holman did not propose the term, others have referred to this hydraulic mechanism as they'e/ effect.
Aortic Valve Stenosis with Aortic Dissection Aortic dissection associated with stenotic aortic valves was reported in four cases by McKusick et al. [52] and six cases by Fukuda et al. [53]. Additional single cases have been reported by Heath [54], Flanders et al. [55], Becker et al. [56], and Gwinn et al. [57], the latter in a 12-year-old boy. Roberts [58] has emphasized the contribution of congenital aortic valve disease to aortic stenosis: of 105 patients with isolated aortic valve stenosis, 54 (51.4 percent) had bicuspid and 13 (12.4 percent) had unicuspid valves, suggesting that congenital valve disease is the most common cause of isolated aortic stenosis. These observations are supported by those of Fenoglio et al. [59] who found that the frequency of calcific stenosis in bicuspid aortic valves increased with age. Stenosis was present in only 28 percent of patients 20 years of age or older but rose to 46 percent over the age of 50 and to 73 percent in those over the age of 70. The stenotic valves usually were obstructed by nodular calcareous masses and less frequently by commissural fusion.
The cystic medionecrosis encountered within the aorta in each case of aortic stenosis and aortic dissection reported by McKusick et al. [52] was attributed to the hemodynamic disturbance. Subsequently McKusick [60] suggested that cystic medial necrosis associated with bicuspid aortic valve and coarctation of the aorta may share a common origin as a developmental defect in the arterial tree, reminiscent of Abbott's proposal in 1928 that the medial lesion is congenital [61].
Bicuspid Aortic Valve
Bicuspid deformity of the aortic valve, currently considered the most common congenital cardiac abnormality, is found in 1.8 percent of autopsies [62]. Functionally, a bicuspid valve is mechanically inefficient and slightly stenotic, even though the leaflets appear normal. In order for such a valve to open, the aortic wall must either be deformed (i.e., pulled inward) or the valve leaflets must be redundant.
Gore [63] found that bicuspid aortic valves were common in individuals with
aortic dissection below the age of 40, occurring in 9 of 32, or 28 percent, of the cases. In a series of 119 aortic dissections reviewed by Edwards [64], 11, or 9 percent, had congenital bicuspid aortic valves; 5 of the 11 were 29 years old or younger. The frequent association of bicuspid aortic valves with coarctation of the aorta has been noted by both Abbott [65] and Reifenstein et al. [66], occurring in 23.5 percent of the former and in 42 percent of the latter cases. Abbott found that bicuspid aortic valves were even more frequent (51.5 percent) in those cases of coarctation which developed rupture of the ascending aorta and which, in most cases, led to an aortic dissection. The contribution of bicuspid aortic valves to aortic stenosis has been previously mentioned; its association with Turner's syndrome is described in a subsequent section.
Coarctation of the Aorta
Abbott's classic review of 200 cases of adult coarctation [65] revealed that 38, or 19 percent, died from aortic rupture which in most cases was associated with aortic dissection. Thirty-three involved the proximal aorta and only five began at or distal to the coarctation. Reifenstein et al. [66] tabulated 104 instances of coarctation published subsequent to Abbott's study and found that rupture occurred in 24 (23%) with involvement of the ascending aorta in 19 and the aorta distal to the coarctation in 5. Only 11 cases of coarctation were encountered among 505 dissections culled from the English literature by Hirst et al. [17] from 1934 to 1954; the 7 cases which occurred below the age of 40 comprised 9 percent of the 74 dissections encountered in this age group. In childhood, coarctation is an important predisposing factor in aortic dissection; 6 of 15 dissections occurring in children 16 years of age or younger had coarctation and 1 additional patient had a hypoplastic aorta [67]. While associated hypertension appears to be a major factor contributing to aortic dissection in patients with coarctation, the occurrence of dissection distal to coarctation suggests that the hydrodynamic effects of stenosis, i.e., post-stenotic dilatation, may also be important. Thirteen percent of the "ruptures" in Abbott's series and 21 percent in Reifenstein's study occurred distal to the site of the coarctation.
Turner's Syndrome
Turner's syndrome, occurring in women with short stature, retarded sexual development, webbed neck, and increase in the carrying angle of the arm is frequently associated with coarctation. Seventeen of eighty, or 21 percent, of patients with Turner's syndrome were found to have coarctation of the aorta in two studies [68,69]. Nora et al. [70] observed that coarctation was limited to the common XO form of the disorder. Eleven of their sixteen patients, or 69 percent, with this genotype had coarctation. While hypertension is usually found in patients with coarctation, 30 percent (14 of 47) of those without coarctation had elevated arterial pressure and may explain why aortic dissection in Turner's syndrome is not limited to those with coarctation.
Giant Cell Aortiti*
Giant cell aortitis, a rare inflammatory condition of the aorta, has a variety of names, including granulomatous aortitis, chronic diffuse mesaortitis, or temporal arteritis with involvement of the aorta [71]. The ascending aorta and arch are the usual sites of predilection, but branches of the aortic arch may also be involved. Clinical syndromes associated with the disease include aortic aneurysm [72], aortic insufficiency [73], or aortic dissection. While the nature of the disease remains obscure, the favorable response of giant cell arteritis in the temporal arteries to adrenal corticosteroids suggests that the disease may be an autoimmune process. Most cases involve patients older than 50 years. Aortic dissection may develop rarely from giant cell aortitis.
We have been able to find seven well-documented cases of aortic dissection in association with giant cell aortitis (Table 2-2). Two other cases with intimal tears in the ascending aorta, but lacking intramural dissection for more than a millimeter or two, have been excluded [78,79]. Two cases were excluded for lack of an autopsy description [80,81]. In each of the seven cases, dissection began in the ascending aorta and often extended to the arch. In one, dissection extended to the level of the inferior mesenteric artery as well. Histologically the lesions were characterized by infiltration of lymphocytes, plasma cells, and a variable number of giant cells of the foreign body type in the outer media. Degeneration of elastic laminae and muscle were equally frequent, each occurring in four cases. Lesions have not shown a relation to changes in the vasa vasorum or to accumulations of mucopolysaccharide within the media. Neither proliferative changes in the vasa vasorum, such as are seen in syphilitic aortitis, have been encountered, nor have spirochetes been found with Levaditi stains. In none of the eight cases were the temporal arteries involved, but this may represent a failure to study the arteries rather than lack of involvement. Headache may have been evidence of temporal artery involvement in the case reported by Ainsworth and Gresham [75]. Involvement of small arteries of the face, such as the lingual artery, was encountered in a case reported by Harris [77].
Syphilitic Aortitis
Syphilitic aortitis might be suspected to contribute to the development of aortic dissection since it produces a mesaortitis with focal destruction of elastic tissue and muscle and typically shows an extensive although often focal chronic inflammatory reaction with plasma cells and lymphocytes predominating. Healing is associated with the formation of well-vascularized scars which tend to extend through the media. Such scars may well limit the spread of aortic dissection as has been documented in a number of reported cases [82-84]. Thus, instead of predisposing to dissection, the well-developed lesions of syphilitic aortitis may actually protect against this catastrophe.
A similarity in frequency of medial lesions encountered in the aorta with and without dissection does not necessarily imply that the lesions are unimportant in the pathogenesis of dissection but rather that in most cases an exciting or precipitating factor is necessary in addition to the presence of a predisposing lesion. Those without medial lesions are quite resistant to dissection. Hypertension appears to be the most common precipitating event and is known to be present at the onset of most cases of aortic dissection. Patients with Marfan's syndrome appear to be an exception to this rule and commonly develop aortic dissection in the absence of hypertension. In vitro studies [91] have suggested that rupture of the vasa vasorum into a solitary focus of degeneration in the outer media may be an adequate stimulus to initiate an extensive dissection of the aorta. In such cases, even multiple sections of the dissected aorta may fail to reveal the site of origin.
FAMILIAL AORTIC DISSECTION
Instances of familial aortic dissection must be critically evaluated, searching for the existence of some known heritable connective tissue disorder such as Marfan's
or the Ehlers-Danlos syndrome. Since Marfan's syndrome was not known to predispose to aortic dissection prior to 1943 [38], no doubt this association was overlooked in many of the earlier reports. It is not known whether Marfan's syndrome was present in the occurrence of aortic dissection in a father and son reported by Fleming and Helwig in 1941 [92] or in the 34-year-old mother and her 14-year-old daughter reported by Griffiths et al. [93], but the latter authors supplied the intriguing information that both were "delicate physically." Aortic dissection was reported by Whittaker and Sheehan in 1954 [94] in a father and son in association with Marfan's syndrome.
The occurrence of aortic dissection in two sisters and one son in the absence of stigmata of Marfan's syndrome was encountered by Hanley and Jones [95]. The aortic lesion typical of Marfan's syndrome, cystic medionecrosis, was present in all three. While the authors propose that cystic medionecrosis may be transmitted as an autosomal dominant trait, an alternate possibility exists that the patient had a forme fruste of Marfan's syndrome with cardiovascular manifestations in the absence of skeletal or ocular features of the disease. In such cases, a history of familial transmission may be the only clue to the correct diagnosis.
Aortic dissection has been reported in the Ehlers-Danlos syndrome, which is characterized by hyperextensile skin ("India rubber man"), hypermobile joints, fragile tissues with easy bruising, and a bleeding diathesis. Although McKusick [96] reported 4 cases considered to have dissection of the aorta, lesions were described only in cases 3 and 4. In case 3, aortic dissection developed in a 14-year-old boy at night while in bed. Case 4, a chronic dissection occurring in a 53-year-old man, was diagnosed by aortography. His 56-year-old sister died following surgical repair of a chronic dissection of the aorta. Their mother had died suddenly at age 56, possibly from a rupture of the aorta. Histological studies revealed lesions of cystic medionecrosis (i.e., elastic tissue lesions) indistinguishable from those seen in classical examples of Marfan's syndrome. Recent studies indicate that those patients with the Ehlers-Danlos syndrome who have a pro- pensity to gastrointestinal or aortic rupture have a deficiency in type in collagen, which normally predominates in the gastrointestinal tract and vascular connective
tissues [97).
RARE CAUSES OF AORTIC DISSECTION
Relapsing Polychondritis
One instance of aortic dissection and six with aortic insufficiency have been reported in relapsing polychondritis, a systemic illness associated with destruction of cartilaginous tissues throughout the body [98]. Diagnostic criteria include recurrent inflammation of at least two cartilaginous sites; involvement of organs of special sense, especially the nose and ears, and biopsy findings compatible with the disease. The disease bears some similarity to the experimental disease in rabbits produced by intravenous injections of papain, i.e., collapse of cartilage and aortic lesions; but the aortic lesion in the rabbit consisted of cartilaginous and bony metaplasia of the media of the aorta [99]. Others [100] have produced aortic dissection in rabbits by intraperitoneal injection of papain.
Juvenile Nephropathic Cystinosis
Strayer [101] reported aortic dissection in a 7-year-old boy with juvenile nephropathic cystinosis, a rare disease transmitted as an autosomal recessive. The author was reluctant to attribute dissection to the mild cystic lesions encountered in the media of the aorta at autopsy. Hypertension with a recorded blood pressure as high as 200/150 appears to have been the major contributing factor.
TRAUMA External
Because the trauma to which an aortic dissection has been attributed has not always seemed relevant, we propose the following criteria as guidelines for considering injury as an etiologic factor: (1) the injury should involve the portion of the body in which the affected segment of the aorta lies; (2) there should be a reasonably close relationship between the time of onset of injury and the development of clinical evidences of aortic dissection; and (3) aortic dissection should be documented by roentgenography or autopsy. Using the above criteria, some of the published cases of "traumatic" dissection fail to qualify; including die three reported by Pierce [102].
In a review of 505 cases, Hirst et al. [17] were able to find only a single instance in which dissection seemed to show a direct relation to trauma. In this instance, the symptoms of aortic dissection developed immediately following an automobile accident in which the driver's chest was compressed between the steering wheel and the back of the seat [103]. Bornstein [104] reported an instance of aortic dissection due to trauma, i.e., an automobile accident. However, both the gross and histological features favor traumatic rupture over true dissection; a circumferential
tear originated just beyond the aortic arch and nearly encircled the aorta at precisely the site where most ruptures due to blunt external trauma occur. The single microphotograph demonstrates transverse rupture of intima and media rather than intramural dissection. Traumatic rupture of the aorta occurs most frequently at a site just distal to the origin of the left subclavian artery [105] and less frequently in the ascending aorta, a distribution which is just the reverse of spontaneously occurring aortic dissection.
A small number of dissections in the abdominal aorta have been due to external trauma. Ngu and Konstam [106] reported the first instance. By 1973, Blute and Ferris [107] were able to collect nine cases from the literature, adding one of their own.
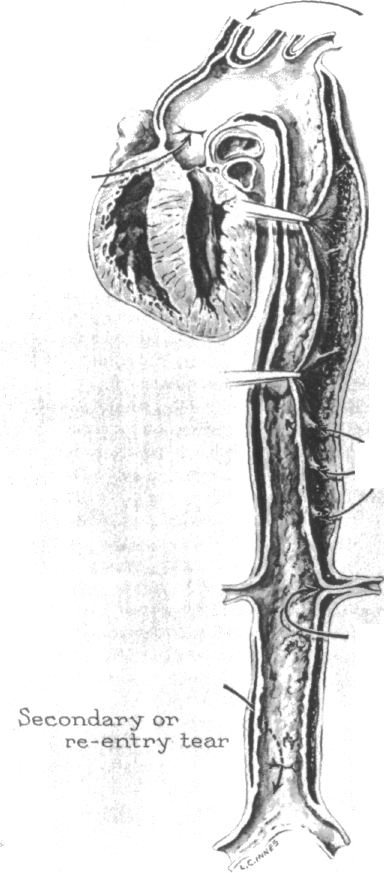
PATHOLOGY OF AORTIC DISSECTION Intimal Tears: Primary vs. Secondary
Aortic dissection most frequently begins in the ascending aorta a short distance above the aortic valve (Figure 2-4). The presence of a tear probably reflects. however, an underlying weakness if not actual disruption of the underlying media, the chief supporting layer of the aorta. The intimal tear in the ascending aorta is usually referred to as a primary or entry intimal tear in order to distinguish it from the secondary or reentry tear which occurs at a more distal site.
Primary tears are located in the ascending aorta in about 62 percent of the cases (Table 2-4) [17]. The tears occur with decreasing frequency as the distance from the aortic ring increases (Table 2-5). Over 50 percent of the tears are located within the first 2 centimeters of the ascending aorta. Primary intimal tears are about five times more likely to be transverse or circumferential in orientation than longitudinal. Tears have taken a variety of shapes in the ascending aorta, including elliptical, oblique, zigzag, or round. The preponderance of transverse intimal tears may reflect the predominantly circular arrangement of the supporting elements of the media [6], but have also been attributed to an elongating type of stress [138]. Intimal tears seldom occur through atheromatous plaques: the likelihood was only 2 percent in the series of cases reported by Shennan [85] and 4.5 percent in the cases reported by Hirst et al. [17].
Next to the ascending aorta, the isthmus of the aorta, the site of attachment of the obliterated ductus (ligamentum arteriosum), is the most frequent site of entry tears. The relative fixation of the aorta has been considered the most likely ex-
planation for tears in this region (see Chapter 3). Tears at the isthmus, like tears in
the ascending aorta, are more frequently oriented transversely to the long axis of the aorta in a 5:1 preponderance. Other sites of intimal tears in decreasing order of frequency include the descending thoracic, aortic arch, and abdominal aorta (Table 2-4). Multiple primary tears occur in about 8 percent of cases.
Secondary or reentry intimal tears are usually situated at a site some distance from the primary intimal tear (Figure 2-5). The iliac arteries have been the most frequent site for a reentry tear, the left and right being involved with about equal frequency. Other sites for secondary tears in decreasing frequency include the lower abdominal aorta and the descending thoracic aorta, particularly the upper segment.
Secondary or reentry tears were only one-sixth as frequent as primary tears (66 vs. 398) in the study by Hirst et al. [17]. Such tears allow the dissecting column of intramural blood to reenter the lumen, often relieving the compression of arteries which originate from the aorta. As noted by Shennan [85] and others [17], the prognosis is considerably improved by a reentry site. Early attempts at the surgical treatment of aortic dissection utilized creation of an artificial reentry site into the lumen, but the procedure proved to be only of temporary benefit.
The primary intimal tear usually communicates with a stratum between the middle and outer thirds of the media, the site of predilection for aortic dissection. This is precisely the depth in the aortic media to which the vasa vasorum normally extend; their rupture will initiate a medial hematoma in this stratum. Aortic dissections tend to extend distally, due to the pulsating force of the blood [139].
As dissection extends over the arch of the aorta, one or more of the three large vessels arising from the arch may be compressed by intramural hemorrhage (Figure 2-6). Dissection may continue down the descending thoracic aorta on the lateral or anterior wall without serious effect on aortic branches.
Involvement of the posterior aspect of the descending thoracic aorta may produce compression with or without sequential shearing of one or more intercostal arteries so that these vessels originate from the dissecting channel. Such severed channels, like an aortic reentry site, may reduce pressure in the intramural channel and favor survival.
Origin of the majority of primary intimal tears close to the aortic valve leaflets is of great functional significance to the aortic valve, since sagging, displacement, tearing of a valve leaflet, or dilatation of the aortic valve ring may result in aortic insufficiency and left ventricular failure (see Chapter 4).
Compression of Aortic Branches
The frequency of involvement of various segments of the aorta as well as the frequency of dissection of branches of the aorta in 450 autopsies [17] is shown in Figure 2-7. Involvement of the iliac arteries (common, external, or internal) was most frequent, and was followed by involvement of the brachiocephalic, the left common carotid, left subclavian, and left coronary artery.
In 1973 Gore and Hirst [140] reanalyzed the data from a previous study [17]
relating the involvement of peripheral arteries to sites of dissection in the aorta. These data (Table 2-6) reveal the natural tendency for dissection to propagate distally rather than proximally. Thus, proximal dissection into the coronary arteries or branches of the aortic arch is largely limited to dissection beginning in the ascending aorta. Dissections arising in the arch or descending thoracic aorta are most likely to produce dissection (and therefore occlusion) of aortic branches distal to the site of origin of dissection. In the most extensive dissection on record, dissection extended as far down as the anterior and posterior tibial arteries [138].
Sites of External Rupture
The site of external rupture of an aortic dissection is to a large extent determined by the location of the primary tear (Table 2-7). Intrapericardial rupture occurred in 70 percent of cases when dissection began in the ascending aorta, but dropped to 35 percent when dissection originated in the aortic arch, 12.3 percent in those beginning in the descending thoracic aorta, and in only 7 percent of dissections with primary tears in the abdominal aorta.
Hemopericardium constitutes the most frequent cause of death in aortic dissection, and is responsible for fatality in about 70 percent of dissections when the patient succumbs in less than 2 weeks; in 20 percent of dissections when the patient survives for 2 to 6 weeks, and in 25 percent of those living longer than 6 weeks [17J.
The volume of the hemopericardium as determined from previously unpublished data on the 505 cases [17] was recorded in 107 cases. Only 12 (11.2 percent) had less than 200 ml; 16 (or 14.9 percent) had 200 to 299 ml; and the remaining 79 (74 percent) had 300 ml or more. The largest amount recorded was 1500 ml. This review suggests that fatality is usually associated with acute accumulations of over 300 ml of blood in the pericardia! sac. Deductions from animal experiments
suggest that the human pericardium can tolerate an acute accumulation of 80 to 100 ml of fluid without symptoms [142].
Next to the pericardium, the pleural cavities are the most frequent site of external hemorrhage with a 5 to 1 (85:17) left-sided preponderance [17]. Relating pleural hemorrhage to the site at which dissection begins, left pleural involvement is found in only 6 percent of the cases starting in the ascending aorta, in 32 percent of the cases originating in the aortic arch, and in 44 percent of those beginning in the thoracic aorta. Only 7 percent of abdominal aortic dissections had pleural hemorrhage. Right pleural involvement lagged behind the left in all but one segment, the abdominal aorta, in which there were too few cases (i.e., only three) to establish a predominance.
Other sites of massive hemorrhage include the mediastinum, retroperitoneum, and the gastrointestinal tract [17].
"Healed" Intimal Tears
In a few cases, primary tears in the aorta have undergone healing ratter than having led to aortic dissection. In such cases, the tear is usually transverse and involves the ascending aorta a short distance above the valve cusps. The resulting lesion has been called an incomplete rupture of the aorta by Peery [143] and incomplete aortic dissection by Schlatmann and Becker [144]. Twelve such cases were encountered in the review of aortic dissection by Hirst et al. [17], and 9 were encountered in addition to 20 complete aortic dissections in the study by Schlatmann and Becker [144]. The latter authors found the lesion to be associated with
disruption of the underlying media. In a case encountered by Hirst and Gore (Figure 2-8), the intimal tear severed the attachment of the commissure between the right and noncoronary cusp, resulting in fatality from intractable aortic insufficiency.
"Healed" Aortic Dissection
T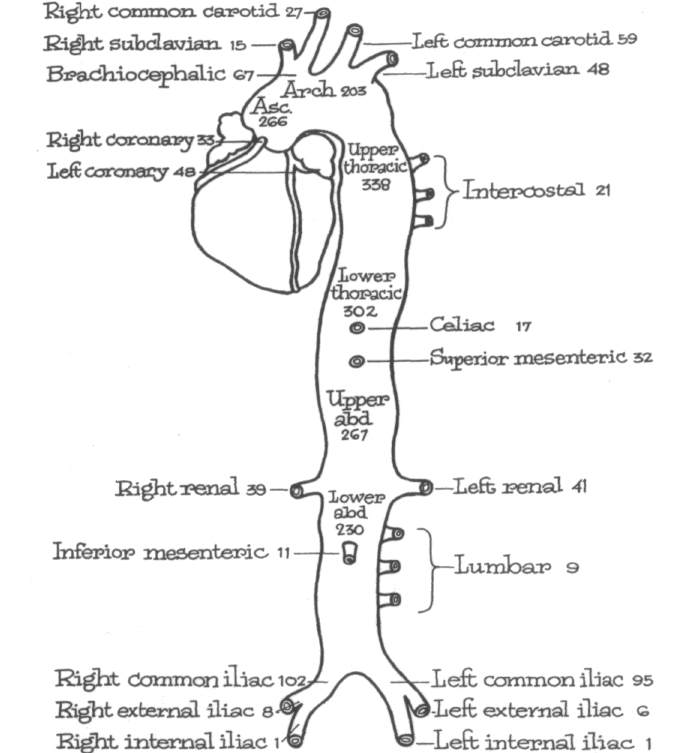 he
patient who survives an acute attack of aortic dissection generally
developsa
so-called double aorta in which a second lumen lies external or
adjacent to the original lumen. Shennan [85] collected 79 examples of
such "healed" aortic dissection.
Healing is usually associated with development of a highly cellular
"pseudointima"
on both the inner and outer lining of the dissecting channel. Healing
is said to have occurred when the dissecting channel is
endothelialized (Figure
2-9). Usually such double aortas have a proximal entry site at the
origin and
a reentry site at the termination of the healed dissecting channel.
Healing by organization
and obliteration of the dissecting channel has been reported in rare
he
patient who survives an acute attack of aortic dissection generally
developsa
so-called double aorta in which a second lumen lies external or
adjacent to the original lumen. Shennan [85] collected 79 examples of
such "healed" aortic dissection.
Healing is usually associated with development of a highly cellular
"pseudointima"
on both the inner and outer lining of the dissecting channel. Healing
is said to have occurred when the dissecting channel is
endothelialized (Figure
2-9). Usually such double aortas have a proximal entry site at the
origin and
a reentry site at the termination of the healed dissecting channel.
Healing by organization
and obliteration of the dissecting channel has been reported in rare
instances [145]. While the minimal period required for endothelialization is not known, this healing reaction was observed within 20 months in a case reported by Conston [146] and in 23 months in a case reported by Weiss et al. [147].
A remarkable feature of healed aortic dissection is the rapid acquisition of atheromatous changes often exceeding in a matter of months the atheromatous changes acquired in a lifetime in the intima of the aorta (Figure 2-10). Healing by endothelialization was said to have occurred in 36 of the 505 cases reported by Hirst et al. [17], and 16 were said to show early to mild atheromatous changes. Patients with chronic aortic dissection often present symptoms and signs of chronic congestive heart failure. If, in addition, there is clinical evidence of aortic insufficiency, syphilitic aortitis may be erroneously suspected to be the cause.
PATHOGENESIS OF AORTIC
DISSECTION
Myron W. Wheat, Jr.
The two basic underlying factors which set the stage for the development of an acute aortic dissection are medial degeneration and arterial hypertension. Medial degeneration is probably the more important of the two, as indicated by acute dissections occurring in normotensive younger patients such as those patients with Marfan's syndrome or hereditary connective tissue defects without Marfan's stigmata [1-6]. A concept of the mechanism of development of aortic dissection is important not only in understanding the disease process itself but even more important in establishing a meaningful approach to the correct treatment. The pathogenesis can be divided into (1) the basic setting, i.e., the patient with medial degeneration with or without systemic arterial hypertension; (2) the intimal tear; (3) propagation of the dissecting hematoma; (4) healing of the dissecting hema-toma with survival or rupture with death of the patient.
THE INTIMAL TEAR
Some authors have stated that acute aortic dissection is the result of rupture of the vasa vasorum in the media of the aorta [7]. The rupture of the vasa vasorum is said to produce bleeding into a previously diseased area of medial degeneration, resulting in a hematoma in the aortic wall. The intimal tear is believed to be secondary to the intramural hematoma. Although there are well-documented instances in which intramural hematomas have occurred without demonstrable intimal tears [7], such an explanation seems unlikely in most cases for the following reasons:
1 The intraluminal pressure in the aorta is always significantly greater than that in the vasa vasorum. The peak systolic pressure in the vasa vasorum is probably no greater than 50 to 60 mmHg, significantly lower than the diastolic Displaced Intimal Calcification The normal aortic wall thickness is 2 to 3 mm [14,34]. The medial hematoma of an aortic dissection obviously increases this measurement. Wall thickness of greater than 4, 5, 6, or 10 mm has been advocated as an indication of the presence of an aortic dissection (Figure 5-7) [24,31,34,39-41]. The most widely accepted figure is 10 mm. The thickness of the aortic wall on a plain chest film can be determined only when the intima is calcified. This increase of distance from intimal calcification to outer aortic soft-tissue border has been emphasized by many authors [15,18,24,37].
This measurement can be misleading, however, for five different reasons. First, the apparent outer soft-tissue border of the aorta can actually be secondary to adjacent paraaortic fat, neoplasm, or hemorrhage [12,29,42,43]. Secondly, the wall itself may be thickened from aortitis [29,42,43].
The third possibility occurs when the intimal calcification and external contour of the aorta are not truly adjacent to each other [12,29,44]. Usually, calcific plaques are thin and flat and are seen on plain films only when they are parallel to the x-ray beam. The outer border of the aorta is also imaged by the x-ray beam being
tangential to the aortic wall. The calcification-aortic contour distance is usually most reliable in the descending thoracic aorta where it is nearly perpendicular to the x-ray beam with virtually no aortic foreshortening. This is not true in a frontal view of the aortic arch. Measurements are unreliable because the true contour-producing portion of the aortic arch may be several centimeters farther posterior than the visualized rim of calcification (Figure 5-8). With the arch "opened up" by a left anterior oblique or a lateral projection, this error can be avoided. Calcifications seen on the superior aspect of the aortic arch are more reliable, even if on frontal view [12,29]. Occasionally, calcified intimal plaques are thick and can be seen on plain radiographs when not parallel to the x-ray beam and may give false measurements even in the descending aorta or in the aortic arch in the left anterior oblique or lateral projection.
A fourth situation might occur when an aortic dissection is chronic and calcification exists in the outer portion of the medial hematoma. Radiographically this calcification will be adjacent to the external contour of the aorta and will suggest that dissection is absent (Figure 5-9). This condition cannot be differentiated from the more commonly seen calcified intima of an atherosclerotic aorta (Figure 5-10) [12,29]. If, in addition, the aorta is dilated, an incorrect diagnosis of fusiform
aortic pressure of the average adult patient, particularly one with hypertension. Therefore, even if a localized intramural hemorrhage should occur, a significant hematoma of the aortic media should not develop. The increased pressure from within the aortic lumen would constantly tend to arrest, compress, and/or stabilize the development of such an intramural hematoma.
Since arterial vasa vasorum are present only in the outer third to one-half of the aortic wall, all dissections should originate in the outer one-half of the media. Such a hypothesis does not fit the actual finding that most dissections have intimal tears.
The vasa vasorum hypothesis does not fit with hydrodynamic data demon strating that the point of impact, or site of greatest stress on the aortic wall, is that point nearest the main stream of blood, i.e., intima and inner third of the media [8J.
If most, or all, aortic dissections immediately involved the outer third of the aortic media and adventitia, many more than the actual 3 percent of the patients should succumb abruptly from acute aortic rupture after initiation of the intimal tear. Actually, 3 percent of the patients with acute aortic dissection die abruptly and only 27 percent die within the first 24 hours [7,9-11].
A more likely explanation of the pathogenesis of acute aortic dissection is as follows:
First, consider the normal hemodynamic or hydrodynamic forces acting within the aorta with each heartbeat. At an average of 70 heartbeats per minute, this propagation offerees occurs just over 100,000 times a day or about 37 million times per year. Viewing this situation externally in relation to the heart and thoracic aorta, what occurs with each heartbeat? The heart, which generates the force and motion, is relatively fixed anteriorly by the sternum and rib cage, and posteriorly by the vertebral column. The heart, ascending aorta, and aortic arch are suspended—much like the pendulum of a clock—by the vessels to the head and upper extremities. The thoracic aorta becomes fixed just distal to the left subclavian artery, as it becomes rather firmly attached by the parietal pleura along the left side of the vertebral column. With each beat, the heart moves predominantly from side to side because of the rigid sternum anteriorly and vertebral column posteriorly. This produces a flexing stress in the ascending aorta and flexing just distal to the left subclavian artery, where mobile aorta joins immobile aorta. The extent and frequency of this flexing action can be documented simply by tracing the outline of the thoracic aorta during systole and diastole in left anterior oblique projections of aortograms and then superimposing the two tracings. In studies of 42 aortograms, 95 percent showed definite lateral movement of the ascending aorta. Fifteen aortograms were evaluated for motion just distal to the left subclavian artery, and 74 percent showed definite lateral motion just proximal to where the descending thoracic aorta becomes relatively fixed (Figure 3-1).
Supportive evidence for the tendency of the aortic wall to be disrupted just distal to the left subclavian artery is available from studies of patients suffering deceleration accidents with traumatic rupture of the aorta [12-14]. Most of the disruptions
occur in the area of the isthmus just distal to the left subclavian artery. It is believed that the transverse tears occur in this area of the aorta because it is the junction of mobile aortic arch with relatively fixed descending thoracic aorta. Pressure from the left lung hilum and a "cutting pressure" from the recurrent nerve have also been implicated [12].
The explanation for the higher incidence of intimal tears in spontaneous aortic dissection in the ascending aorta rather than in the descending or abdominal aorta is related to a number of factors. The hemodynamic events occurring inside the aorta with each cardiac systole probably have their greatest effect on the ascending aorta, as shown by the most marked degenerative changes occurring with age in the ascending aorta [15,16]. The ascending aorta is also the area where the most external motion and flexion occur with cardiac systole.
As a result, in certain patients, as a consequence of many (if not all) of the
above-mentioned factors—degeneration of the media, recurring flexion of the aorta, varying forces applied to the aortic intima by the hemodynamic events operating within the aorta—an intimal tear occurs. Sixty to sixty-five percent of the tears arise in the ascending aorta, 5 to 10 percent in the aortic arch, and 30 to 35 percent in the first portion of the descending thoracic aorta. The depth of penetration of the tear into the media and to some degree the distance of propagation of the subsequent dissecting hematoma is related to the extent of degeneration in each individual aortic media.
PROPAGATION OF THE DISSECTING HEMATOMA
Following the intimal tear and the development of a dissecting hematoma, a second set of forces becomes the more important. These forces are those which primarily propagate or continue the dissecting hematoma and include blood viscosity, blood pressure, velocity (or shearing forces), turbulence, and steepness of the pulse wave, dpidt max (see Chapter 7).
 Experimental
evidence indicates that the two most important forces propagating
the dissecting hematoma are (1) the steepness of the pulse wave [8]
and (2) the blood
pressure [17,18]. Therefore, the forces that cause the dissection to
progress and eventually to rupture, leading to the death of the
patient, are originated by the heart
and are also related to peripheral arterial resistance, or afterload.
Experimental
evidence indicates that the two most important forces propagating
the dissecting hematoma are (1) the steepness of the pulse wave [8]
and (2) the blood
pressure [17,18]. Therefore, the forces that cause the dissection to
progress and eventually to rupture, leading to the death of the
patient, are originated by the heart
and are also related to peripheral arterial resistance, or afterload.
SUMMARY OF PATHOGENESIS
The pathogenesis of most aortic dissections can be viewed as follows:
Medial degeneration in the wall of the thoracic aorta sets the stage by de creasing the cohesiveness of the layers of the aortic wall, particularly of the media itself (Figure 3-2a).
Repeated motion of the aorta related to the beating of the heart results in flexion stresses, most marked in the ascending aorta and first portion of the descending thoracic aorta, 60 to 100 times a minute, 37 million times a year.
Hydrodynamic forces in the bloodstream, related to the pulse wave propa gated by each myocardial contraction, as well as the level of the systolic blood pressure, act upon the wall of the aorta—most markedly the proximal ascending aorta.
A combination of these factors eventually results in an intimal tear which leads to a hematoma dissecting into the media of the aortic wall for varying depths (Figure 3-26). Hydrodynamic forces in the bloodstream, primarily related to the steepness of the pulse wave dpldi max, as well as the blood pressure, continue the propagation of the dissecting hematoma (Figure 3-2c) until rupture occurs either (1) back into the lumen of the aorta resulting in a "spontaneous cure," a rare but documented occurrence [19-23], or more likely, (2) rupture into the pericardium or pleural cavity leading to death within 30 days in most instances.
AORTIC DISSECTION:
PRESENTATION
AND DIAGNOSIS
Eve Elizabeth Slater
Although aortic dissection was first described in 1761 by Morgagni, it was not until 1856 that a correct antemortem diagnosis was made by Swaine and Latham [1,2]. Clinical recognition of this disease continued to lag for almost a century. In an extensive review of 734 cases published up to 1950, only 10.6 percent of patients received the proper antemortem diagnosis [3]. Since then, more widespread awareness of this disease and increasingly sophisticated diagnostic techniques, most notably angiography, have allowed accurate and prompt recognition in well over 90 percent of cases. Nevertheless, a high index of suspicion based upon clinical data is still the single most important factor in making the diagnosis and in initiating proper therapy.
