
- •Методическая записка
- •§ 1. Научный и технический тексты
- •Practical Skill
- •Text 1. And the Galaxy Said, Let There Be Light
- •Text 2. Positive Effects of Carbon Dioxide for Plant Growth
- •Самосознание лингвистики - вчера и завтра р. М. Фрумкина Познавательные процедуры в лингвистике и других гуманитарных науках
- •§ 2. Учебник
- •Practical Skill
- •§ 1. Elements, Atoms, and Molecules
- •§ 2. Structure of the atom
- •The word atom is derived from the Greek word atom which means indivisible. The Greeks concluded that matter could be broken down into particles to small to be seen. These particles were called atoms
- •Visualizing Atomic Orbitals
- •A Chemistry Textbook
- •§ 13. Природа Луны
- •§ 3. Инструкция
- •2. Applicability and scope
- •3. Definitions
- •4. Policy
- •5. Responsibilities
- •Filling in a Tax Return
- •1.C. The language of instruction: extracts from An Operating Manuals How to operate your Washamatic
- •1.D. Cardiovascular drugs
- •Adelphane For the treatment of hypertension
- •Indication
- •Инструкции по применению шприц-тюбика
- •2Б. Должностная инструкция переводчика
- •I. Общие положения
- •II. Должностные обязанности
- •III. Права
- •IV. Ответственность
- •§ 4. Энциклопедическая статья
- •Practical Skill
- •Text 3. Spaarndam
- •Text 4. Space Monkeys
- •§ 5. Деловое письмо
- •Practical Skill
- •An Exchange Of Letters
- •2. Dear Mr. Marsh,
- •A Letter Of Application
- •§ 6. Документы физических и юридических лиц
- •Practical Skill
- •§ 7. Траурный информационный текст
- •3. Предложите прилагательные, которые могут быть употреблены со следующими существительными:
- •4. Переведите предложения на английский язык.
- •1. Сопоставьте оригинал Текста 1 и его перевод.
- •Томские новости: Соболезнования томичам пришли из республики Дагестан и Кузбасса
- •§ 8. Газетно – журнальный информационный текст
- •Practical Skill
- •1. Norman Kember in the latest video
- •It is completely inconceivable that all the people in detention in Iraq would be released
- •'Kind of breakthrough'
- •How events unfolded
- •Muslim group's plea
- •2. Two held in Russia-uk 'spy' case
- •3. Pinochet daughter held in Chile
- •'Political persecution'
- •Foreign accounts
- •4. A News Bulletin
- •2.Б. Джеймс Бонд против майора Пронина
- •§ 9. Эссе (художественная публицистика)
- •Practical Skill
- •1. Essay on love
- •Text 2. An Overview of the Hard-Boiled fiction of Murakami Haruki.
- •Text 3. A Feature Article
- •Харуки Мураками Переводить и быть переводимым
- •§ 10. Мемуары
- •Practical Skill
- •Text 1. Ulysses s. Grant (1822–85). Personal Memoirs. I Ancestry—Birth—Boyhood
- •Text 1. Барон де Марбо
- •Война в седле
- •(Избранные главы из воспоминаний)
- •Глава 6: 1799
- •Text 2. Отрывок из мемуаров л. Гурченко «Аплодисменты» я люблю тебя, жизнь
- •§ 11. Научно–популярный текст
- •Practical Skill
- •The Rise and Fall of the Mayan Empire
- •Text 2. Greenhouse Plants? Vegetation may produce methane
- •Text 3. The Continuing Mystery of America's 'Lost Colony'
- •Видим ли мы Вселенную?
- •§12. Юридический текст
- •The Constitution of the United States of America
- •Article I
- •§ 13. Музыковедческий текст
- •1. Musicology
- •Introduction
- •2. 'Folk' Influences
- •3. Words And Music
- •4. Beethoven was a narcissistic hooligan
- •Антонио доменико виральдини (1705-1741) Руфь изначальная и Руфь осмысленная гением. Личность музыканта
- •О музыкальном стиле.
- •Text 2. Ритм-энд-блюз:
- •§ 14. Искусствоведческий текст
- •Practical Skill
- •Text 1. A bather or a nymph? Fon Neff’s painting
- •Text 2.
- •Impressionism
- •Overview
- •Beginnings
- •Impressionist techniques:
- •Content and composition
- •Text 3. Socialist realism
- •Socialist realism in the Soviet Union
- •Socialist realism in other states
- •Characteristics of socialist realism
- •Text 1.
- •Text 2. Архитектура Лондона
- •§ 15. Философский текст
- •Practical Skill
- •Rene Descartes
- •I. The Method of Natural Philosophy
- •I. Rules of reasoning in philosophy
- •Text 2. Why did the chicken cross the road?
- •Философия античности. И.А. Щекалов
- •Философия досократиков
- •§ 16. Проповедь
- •Text 2. Family Focus on Christ
- •Text 3. A Sermon
- •Когда Бога нет
- •§ 17. Рекламный текст
- •Practical Skill
- •1. Реклама духов:
- •2. Реклама автомобилей:
- •3. Реклама чай Earl Grey:
- •4. Реклама косметики и парфюмерии для женщин
- •5. Рекламы консалтинговой компании «Locate in Scotland”:
- •§ 18. Афоризмы, пословицы, заголовки
- •Practical Skill
- •Poles apart
- •§ 19. Библиографические материалы, каталоги и т.П.
- •References
- •Литература
- •Требования к зачету.
Visualizing Atomic Orbitals
The atomic orbitals of the hydrogen atom can be visualized as a cloud around the nucleus. The orbital represents a probability of finding the electron at a particular location. Darker regions signify a greater probability. Shown below are the 1s (lowest orbital and the 2s orbital.
 1s
1s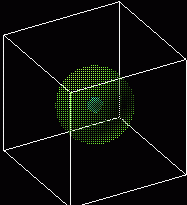 2s
2s
Atomic orbitals do not always have the shape of a sphere. Higher orbitals have very unusual shapes.
1.B. An extract from
A Chemistry Textbook
Nitrogen
Nitrogen was discovered by Rutherford and by Priestley, working independently, in 1772. When free from moisture and carbon dioxide, air contains approximately 78 per cent of nitrogen by volume. Most of the nitrogen used commercially is produced by the fractional distillation of liquid air. This commercial nitrogen is usually supplied with a guaranteed purity of 99.7 per cent. The small amount of oxygen remaining may be removed by passing the gas through a tube containing copper turnings at bright red heat or by washing in chromous chloride solution.
Preparation from compounds
There are various ways of preparing nitrogen gas in the laboratory. In small quantities, extremely pure nitrogen may be evolved by thermal decomposition of sodium or barium azide in a vacuum. Usually it is prepared by the removal of hydrogen from ammonia or its compounds in one or other of the following ways:
Ammonia or a mixture of ammonia and nitric oxide is passed over cupric oxide at high temperature.
Ammonia is passed into a solution of bromine and caustic soda in water. The resultant oxidation of the ammonia releases nitrogen.
The decomposition by heat of a solution of ammonium nitrite, or of a mixture of sodium nitrite and ammonium chloride, yields nitrogen.
Ammonium dichromate decomposes violently on heating, giving off nitrogen and leaving a residue of chromic oxide.
Pure nitrogen gas is colourless, odourless and tasteless. It has slight solubility in water, has no action on litmus and does not turn lime water milky. It does not support combustion or respiration, although it is non-poisonous.
If nitrogen gas at a pressure of approximately 0.1 mm. is subjected to an electric discharge a yellowish-orange glow is emitted. The gas continues to give. off a glow for several minutes after the discharge is switched off. It was suggest by Strutt that this "active nitrogen", as it is called, consisted of the gas in its atomic state, and it has since been demonstrated experimentally that active nitrogen consists mainly of normal molecular nitrogen with an admixture of ground-state atoms. The afterglow is a product of the emission-band spectra of excited nitrogen molecules formed as the result of recombination of single atoms of nitrogen.
Nitrogen compounds: Ammonia
Ammonia is a colourless gas, lighter than air, with a pungent smell. It is easily converted into its liquid state, either by refrigeration or by compression. The solubility of ammonia in water is greater than that of any other gas, 1148 volumes of ammonia being dissolved by I volume of water at 0°, and 739 volumes at 20°. The solution is made by passing ammonia gas into cooled distilled water. All the gas is liberated on boiling.
The Haber process is now most usually used for the production of ammonia. In this process, the constituent elements, hydrogen and nitrogen, in the ratio of 3 volumes to I, are brought together at high temperature and pressure in the presence of a catalyst, generally pure iron mixed with molybdenum or potassium and aluminium oxides. Commercial ammonia prepared by the Haber process contains a small amount of water. This cannot be removed by the usual drying agents, such as sulphuric acid, calcium chloride and phosphorus pentoxide, which all react with ammonia. Drying may be effected, however, by condensing the ammonia in a vessel containing metallic sodium. The sodium dissolves, forming a blue liquid, and reacts with any water present The blue liquid may then be distilled to yield ammonia of a high degree of purity.
Задание № 2. Перед вами отрывок из учебника астрономии. Сделайте предпереводческий анализ. Установите тип информации текста. Выполните фоновый анализ. Представьте письменный перевод.
