
We all know the world has its problems, particularly where the environment is concerned. But not too many of us know the details, and the stories behind those problems. On our website, many environmental problems, effects and disasters are described. Here, these are summarized. The links will bring you to related informational pages.
Acid rain, mist and fog
Acid deposition is a general name for a number of phenomena, namely acid rain, acid fog and acid mist. This means it can imply both wet and dry (gaseous) precipitation. Acid deposition is a rather well known environmental problem, for example acid fog killed several thousand people in London in 1952. Acid deposition is concerned with long-range rather than local effects. Pollutants are mixed in the atmosphere and therefore usually cannot be attributed to any local source. Pollutants are generally more dispersed and of lower concentrations than local ground level pollutants. Acid
deposition typically has a pH below 4, but this may be as low as
1.5 under seriously acidic conditions. It primarily consists of
two types of compounds, namely sulphuric
acid (H2SO4) and nitric
acid (HNO3).
S
|
What is air pollution?
|
Air pollution means the presence of one or more unwanted substances in air. Air pollutants have a negative impacts on humans, animals and plants, and on air quality. The most frequently present categories of air pollutants are sulphur oxides, nitrogen oxides, Volatile Organic Compounds (VOC) and small dust particles (aerosols). |
What causes air pollution?
The main sources of air pollution are the industries, agriculture and traffic, as well as energy generation. During combustion processes and other production processes air pollutants are emitted. Some of these substances are not directly damaging to air quality, but will form harmful air pollutants by reactions with other substances that are present in air. Examples of large-scale air pollutants are VOC (Volatile Organic Compounds) and small dust particles. When large concentrations of these substances are emitted this negatively affects ecosystems, materials and public health. Emissions of nitrous oxide (N2O) mainly stem from agriculture, because nitrogen in soils can easily be denitrified by bacteria. Nitrous oxide is emitted during the denitrification process. Additionally, the application of (artificial) fertilizers causes emissions of ammonia (NH3), nitrogen oxides (NOx) and methane CH4), a greenhouse gas. The agricultural sector is known for its extensive use of pesticides. This application causes emissions of many toxic chemicals. Industrial processes vary greatly and as a result there are many different chemical wastes. The industries are responsible for emissions of carbon monoxide, carbon dioxide, sulphur dioxide, nitrogen oxides, small dust particles, VOC, methane, ammonia and radioactive radiation. During energy generation chemicals such as methane are released into the air as a result of oil and natural gas extraction. The combustion of coal and natural gas for electricity production causes the release of sulphur dioxide, nitrogen oxides andcarbon dioxide into the air. Traffic is held responsible for one-third of the greenhouse gas emissions. Emissions caused by traffic are mainly those of carbon dioxide, carbon monoxide, nitrogen oxides, VOC and small dust particles. Consumers are also partly responsible for air pollution. Firstly because the products they use have caused air pollution during their production and distribution and secondly because heating of houses and offices causes chemicals release into the air. When people use paints or cosmetics VOC is released and perspiration, pet fertilizer use and cleanser use cause ammonia emissions. Last but not least, many chemicals (carbon dioxide, carbon monooxide) are emitted during smoking.
How does air pollution spread and how can we handle this?
The dispersion of air pollutants mainly depends on physical processes is air; those of wind and weather. How far air pollutants are transported mainly depends upon particle size of the compounds and at which height the pollution was emitted into the air. Fumes that are emitted into air through high smoke stags will mix with air so that local concentrations are not very high. However, wind will transport compounds and the pollution will become very disperse. Rain can remove pollutants from air. This causes precipitation and consequentially soil and water pollution. For environmental agencies it is very important to determine exactly how an air pollutant spreads. Air is not a very complex medium. This enables us to predict the dispersion of air pollutants with computer models. In a computer model dispersion is calculated by means of different parameters, such as wind speed, wind direction, temperature, air humidity and cloudiness. These predictions are of great significance when we are dealing with toxic clouds or radioactive radiation, because these are a danger to human health and because inhabitants of polluted areas need to be warned.

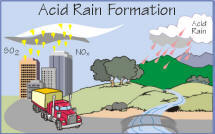 ulphuric
acid is formed by conversion of sulphur dioxide emitted from power
stations, melting processes, home fires, car exhausts and other
sources. It contributes about 70% to the overall acidity of
deposition.
Reaction
mechanism: SO3 +
H2O
-> H2SO4
Nitric
acid is formed from nitrogen oxide (NOx)
emissions from fossil
fuel combustion.
It contributes about 30% to the overall acidity of
deposition.
Reaction
mechanism: NO2 +
OH- ->
HNO3
Acid
rain has various environmental and health effects, for example:
-
Chocking plant leave pores (forest loss)
- Corroding stone
and brick walls of buildings and monuments
- Corroding paper
and rubber objects
- Altering soil chemistry (soil
acidification, loss of plant nutrients)
- Altering the
chemical balance of lakes and streams
- Disrupting fish gill
operation (fish deaths)
- Deteriorating human breathing
disorder (asthma, bronchitis, lung oedema)
When people
die of acid deposition it is usually caused by access mucous
production in the bronchi, leading to chocking from a lack of
oxygen, or a heart attack.
Acid
deposition in various countries
Acid
deposition is a transboundary environmental
problem. This basically means that emissions in one country may
affect forests and structures in a neighbouring country.
Therefore, international agreements were made, such as the Sulphur
emissions Reduction Protocol (1979) and
the Convention
on Long-Range Transboundary Air Pollution (1983).
Some
examples of countries that experience(d) acid deposition, either
from their own sources or from transboundary air
pollution:
- Britain:
smog episodes around London, particularly in 1952
- Germany:
acid mists in central Germany and the Black Forest area, acid cold
smog from Poland and former Czechoslovakia in 1985
- Greece:
intense industrialization in the Athens area causes deterioration
of ancient monuments such as the Parthenon by acid
deposition
- Italy:
damage to Venice structures from acid deposition
- Scandinavia:
15% of acid rain caused by Great-Britain
- Scotland:
episodes of black acid snow in the Cairngorm mountains in
1984
- The
Netherlands:
corrosion of bells of the Utrecht Dom tower since 1951
- United
States:
acid rains disrupts forest ecosystems and pollutes surface waters,
industrial fossil fuel combustion processes are adapted to prevent
sulphur dioxide emissions
ulphuric
acid is formed by conversion of sulphur dioxide emitted from power
stations, melting processes, home fires, car exhausts and other
sources. It contributes about 70% to the overall acidity of
deposition.
Reaction
mechanism: SO3 +
H2O
-> H2SO4
Nitric
acid is formed from nitrogen oxide (NOx)
emissions from fossil
fuel combustion.
It contributes about 30% to the overall acidity of
deposition.
Reaction
mechanism: NO2 +
OH- ->
HNO3
Acid
rain has various environmental and health effects, for example:
-
Chocking plant leave pores (forest loss)
- Corroding stone
and brick walls of buildings and monuments
- Corroding paper
and rubber objects
- Altering soil chemistry (soil
acidification, loss of plant nutrients)
- Altering the
chemical balance of lakes and streams
- Disrupting fish gill
operation (fish deaths)
- Deteriorating human breathing
disorder (asthma, bronchitis, lung oedema)
When people
die of acid deposition it is usually caused by access mucous
production in the bronchi, leading to chocking from a lack of
oxygen, or a heart attack.
Acid
deposition in various countries
Acid
deposition is a transboundary environmental
problem. This basically means that emissions in one country may
affect forests and structures in a neighbouring country.
Therefore, international agreements were made, such as the Sulphur
emissions Reduction Protocol (1979) and
the Convention
on Long-Range Transboundary Air Pollution (1983).
Some
examples of countries that experience(d) acid deposition, either
from their own sources or from transboundary air
pollution:
- Britain:
smog episodes around London, particularly in 1952
- Germany:
acid mists in central Germany and the Black Forest area, acid cold
smog from Poland and former Czechoslovakia in 1985
- Greece:
intense industrialization in the Athens area causes deterioration
of ancient monuments such as the Parthenon by acid
deposition
- Italy:
damage to Venice structures from acid deposition
- Scandinavia:
15% of acid rain caused by Great-Britain
- Scotland:
episodes of black acid snow in the Cairngorm mountains in
1984
- The
Netherlands:
corrosion of bells of the Utrecht Dom tower since 1951
- United
States:
acid rains disrupts forest ecosystems and pollutes surface waters,
industrial fossil fuel combustion processes are adapted to prevent
sulphur dioxide emissions 