
3 Dienes
Dienes are a class of hydrocarbons with the general formula CnH2n-2 , that contain two double bond.
Because they contain fewer hydrogen than related alkanes, dienes, like alkenes and alkynes, are referred to as unsaturated.
Multiple bonds that alternate with sigle bonds are said to be conjugated.
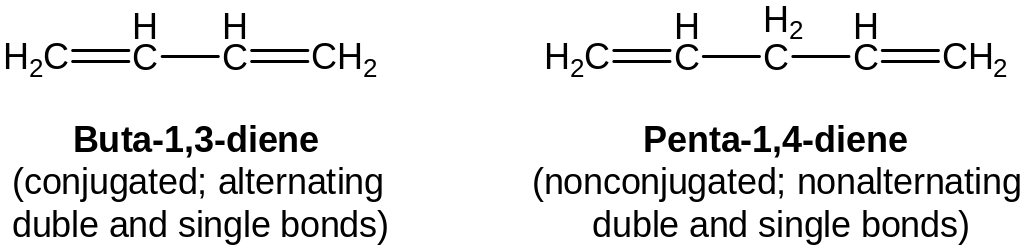
Nonconjugated dienes have properties of alkenes.
Conlugated dienes are similar to other alkenes in much of their chemistry, but there are also important differences.
Conlugated dienes are somewhat more stable than nonconjugated dienes .
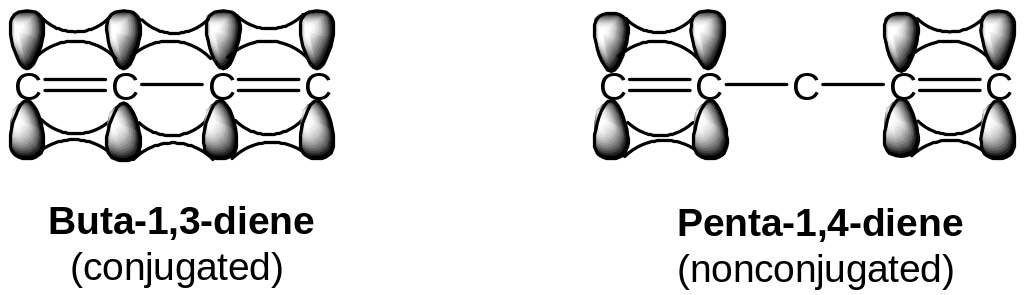
The π electrons are spread out, or delocalized, over the entire framework rather than localized between two specific nuclei.
Electron delocalization always leads to lower energy and greater stability of the molecule.
3.1 Preparation of Dienes
Industrially Preparation
Dienes can be prepared industrially by high-temperature catalytic dehydrogenation of alkanes or alkenes over a chromium oxide/aluminum oxide (Cr2O3/Al2O3) catalyst.

Dienes can be prepared industrially by acid-catalyzed (or Al2O3-catalyzed) double dehydration of dioles.
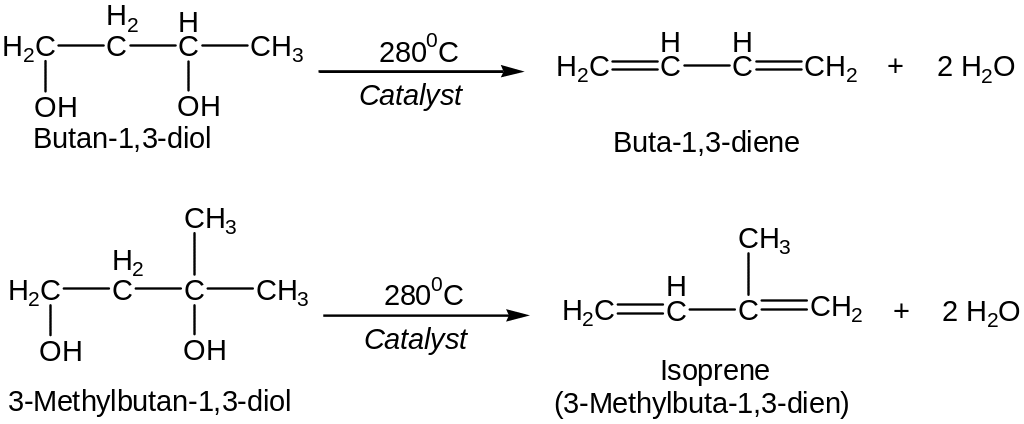
Dehydration of unsaturated alcoholes.

Catalytic dehydrogenation and dehydration of ethanol. (Lebedev’s reaction) over a zinc oxide/aluminum oxide (ZnO/Al2O3) catalyst.
![]()
3.2 Reactions of Dienes
Conjugated dienes undergo two reactions not observed for nonconjugated dienes:
Electrophilic addition reaction;
Diels – Alder cycloaddition
Electrophilic Additions to Conjugated Dienes: Allylic Carbocations
Addition of HX
When conjugated diene is treated with an electrophile such as HBr, mixtures of 1,2 and 1,4 aducts are formed

When buta-1,3-diene is protonated, two carbocation intermediates are possible: a primary and a secondary allylic cation (allylic means “next to a double bond”)
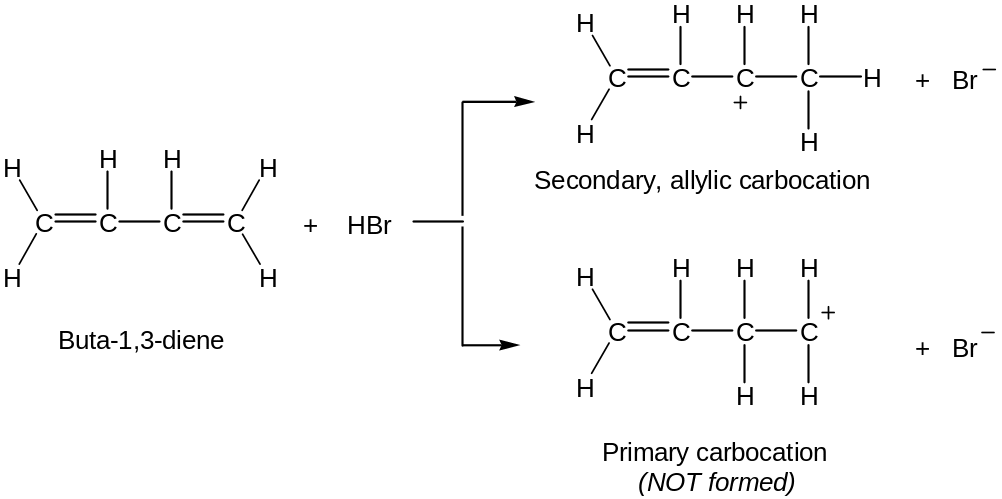
Since an allylic cation is stabilised by resonanse, it is more stable and forms faster than a less stable primary carbocation
An allylic cation has two resonance forms:
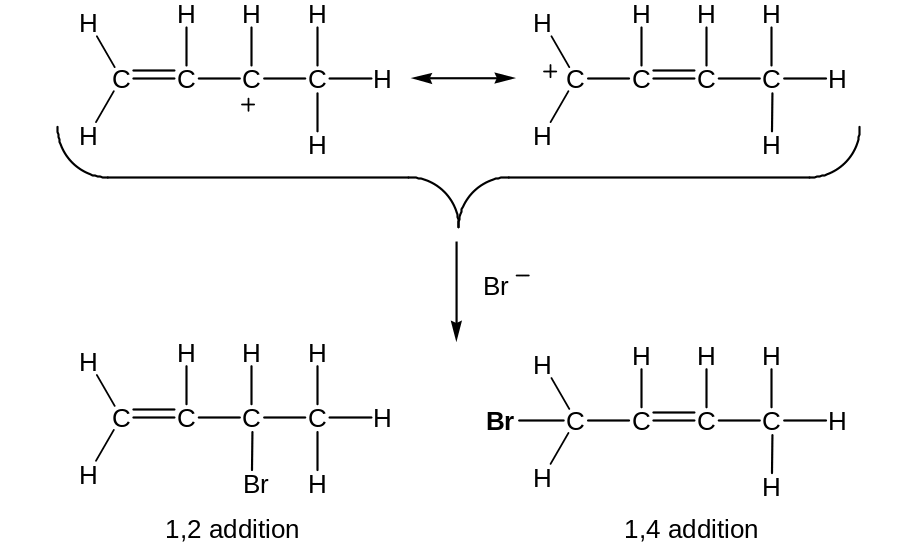
Thus, a mixture of 1,2 and 1,4-addition products results.
Addition of X2
Many other electrophiles besides HBr add to conjugated diene and mixtures of products usualy formed.
For example:

The Diels–Alder Cycloaddition Reaction
Conjugated diene react with electron-poor alkenes or alkynes (dienophiles) in a single step through a cyclic transition state to yoeld a cyclohexene or cyclohex-1,4-diene

The two alkene (alkyne) carbones and carbon 1 and 4 of the diene regybridized from sp2 (sp) to sp3 (sp2) to form two new single bonds. Carbons 2 and 3 of the diene remain sp2-gybridized to form the new double bond.
The Dienophile
The Diels-Alder reaction occurs most rapidly and in highest yield if the dienophile has an electronwithdrawing substituen group (its have negative resonance effect, π,π-conjugation)

The Diels-Alder reaction is stereospecific: The stereochemistry of the starting dienophile is maintained during the reaction, and single product stereoisomer results.

The Diene
Only in the s-cis conformation (“cis-like” about the single bond) are carbons 1 and 4 of the diene close enough to react through a cyclic transition state.

Some dienes can’t adopt an s-cis conformation and therefore don’t undergo Diels–Alder reaction.
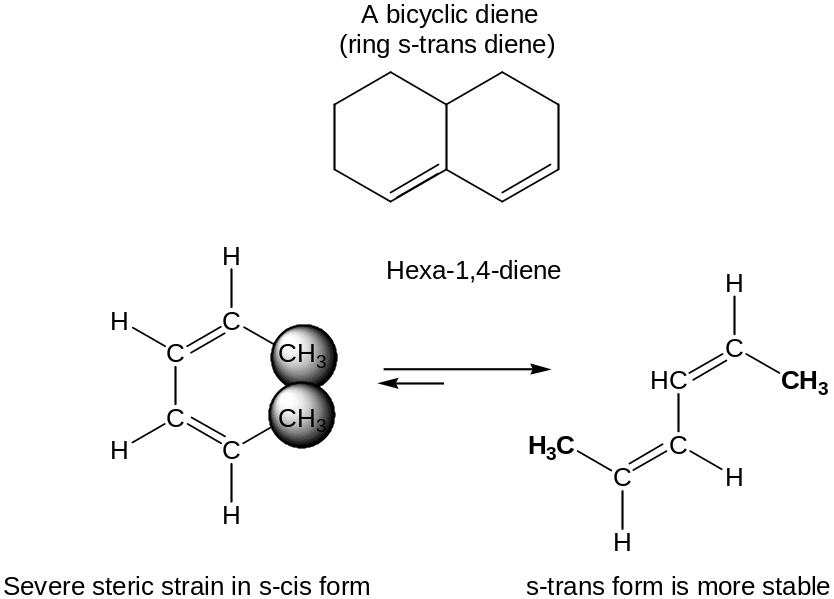
Diene Polymers: Natural and Synthetic Rubbers
Diene polymers are sructurally more complex than simple alkene polymers, because double bonds remain every four carbon atoms along the chain, leading to the possibility of cis-trans isomers.
The reaction can be initiated either by radical, like ethylene polymerization, or by acidic catalyst
Polymerization is a 1,4 addition of the growing chain to a conjugated diene monomer

Rubber is a naturally occurring cis polymer of isoprene.
Gutta-percha trans isomer of natural rubber, also occurs naturally.
Harder and more brittle than rubber, gutta-percha has a variety of minor application, including occasional use as the covering on golf balls.

Both cis- and trans-polyisoprene can be made, and the synthetic rubber thus produced is similar to the natural matarial.
Neopren, an exelent, though expensive, synthetic rubber with good weather resistance, is used in the production of industrial hoses and gloves, among other things.
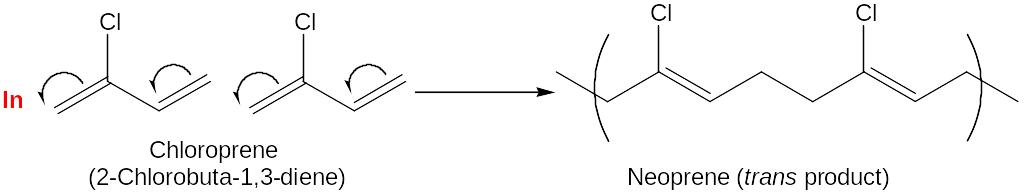
Both natural and synthetic rubbers are soft and tacky unless hardened by vulcanization.
Vulcanization involves heating the crude polymer with a few percent by weight of sulfur.
Sulfur forms bridges, or cross-links, locking the chains together into immense molecules that can no longer slip over one another
The result is a much harder rubber with greatly improved resistance to abrasion.

Questions and problems
Write the formula of unsymmetrical disecbutylethylene and give its name using the IUPAC nomenclature.
Write the formula -ethyl--secbutylethylene and give its name using the IUPAC nomenclature.
F
 ind
out the structure of compound if empirical formula and the
reactions’ products are known: С8Н16,
while ozonolisys butanal is created
СН3
– СН2-СН2-
СНО
.
ind
out the structure of compound if empirical formula and the
reactions’ products are known: С8Н16,
while ozonolisys butanal is created
СН3
– СН2-СН2-
СНО
.
What compounds can be used to receive the compound while the Diels-Alder reaction. Write the equations. A) hexa-1,3-diene; dicianoacethylene; B) hexa-2,4-diene; tetracianoethylene; C) cyklopentene; cianoethylene; D) cyklohexadiene; tetracianoethylene.
Make the synthesis of 4,4,5,5-tetramethylhex-2-ene from 3,4,5,5-tetramethylhex-1-ene
Write the reaction of addition of HBr to 3,4,4,5,5-pentamethylhex-1-ene: without peroxides. What is the mechanism of this reaction?
Choose the right way of synthesis of 2-methyl-3,3-dibromobutane. Write the equations. А)
 В)
В) С)
С)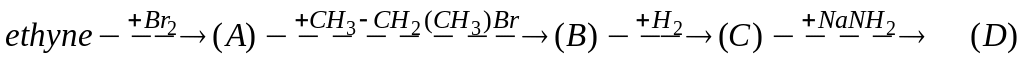 D)
D)
From what alkyne 4,4-dimethylpentan-2-on can be receved in the conditions of the Kucherov’s reaction.
