
2. Alkynes
Alkynes are hydrocarbons with the general formula CnH2n-2 that contain a carbon – carbon tripl bond.
Because they contain fewer hydrogen than related alkanes, alkynes, like alkenes, are referred to as unsaturated
Acetylen, H-C≡C-H, is simplest alcyne

2.1. Nomenclature of Alkynes
Determine the stem name by selecting the longest possible straight chain containing the C ≡ C triple bond and use the ending ‘-yne’
Number the parent chain so as to include both carbon atoms of the triple bond, and begin numbering with the end of the chain nearer the C ≡ C triple bond
Designate the position of the C ≡ C triple bond by using the number of the first atom of the triple bond
Designate the positions of the substituents by using the numbers obtained by application of rule 2
Examples:
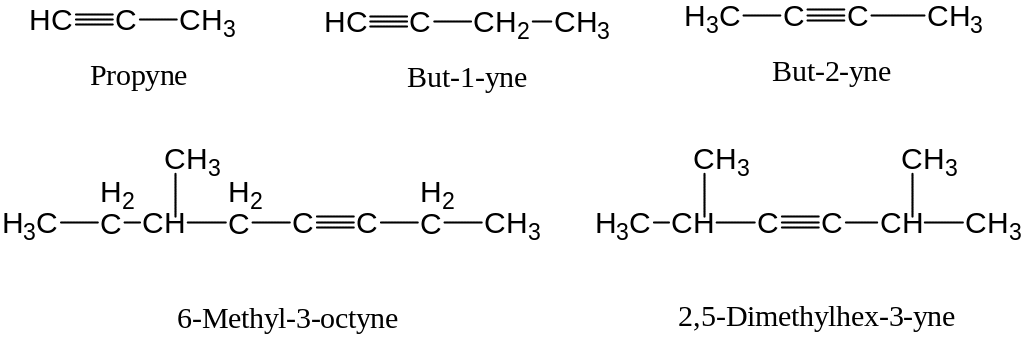
Compounds with more than one triple bond are called diynes, triynes, and so forth;
Compounds containing both double bonds are called enynes (not ynenes). Number of an enyne chain starts from the end nearer the first multiple bond, whether double or trihle. When there is a choice in numbering, double bond receive lower number than triple bond
e.g.

As with hydrocarbon substituents derived from alcanes and alkenes alkynyl groups are also possible e.g.

2.2 Physical Properties of Alkynes
At R.T., C1 – C3: gases ; C4 – C15: liquids ; > C16: waxy solid
Higher members have higher melting points (Increase in molecular mass Þ Increase in intermolecular force)
Branched-chain alkynes have lower boiling points than straight-chain alkуnes (molecule is more compact Þ surface area ¯ Þ van der Waals’ force ¯ Þ boiling point ¯)
2.3 Preparation of Alkynes
Industrially Preparation
Аlkynes can be prepared industrially by high-temperature decomposition (pyrolysis) of methane.
![]()
Acetylen preparation from calcium carbonate
![]()
Alkanes and alkenes dehydrogenation
![]()
Elimination of a hydrogen halide molecule from vicinal dihalide
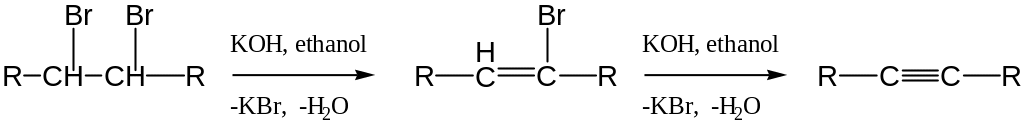
Acetylide ion alkylation

2.4 Reactions of Alkynes
Alkynes and alkenes have similar reactivity
The carbon-carbon single bond in ethane has a strength of 376 KJ/mol, the carbon-carbon double bond of ethylene has a strength of 611 KJ/mol and the carbon-carbon triple bond of acethylene has a strength of 835 KJ/mol
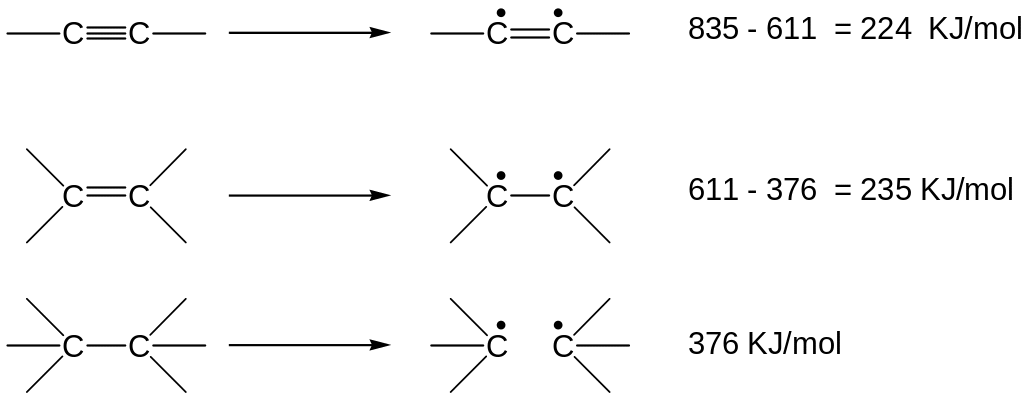
Values of energy needed to break alkyne and alkene π bonds are similar Þ alkynes undergo addition reactions
Electrophilic Addition Reactions
Addition of HX
Addition of HX to C ≡ C triple bond yields a dihalide (in excess of acid)
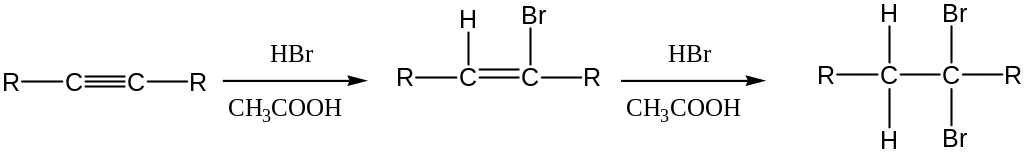
After addition of 1 equivalen of HX, trans stereochemistry of H and X is normally (though not always) found in the product.

The mechanism for the addition of HBr to an alkynes is similar to that of addition to an alkenes follows Markovnikov’s rule

Addition of X2
Bromine and chlorine also add to alkynes to give addition products, and trans stereochemistry again results, after addition of 1 equivalen of X2 :

Mercuric Ion-Catalyzed Hydration (Kutcherov’s reaction)
Alkynes can’t be hydrated as easily as alkenes because of their lower reactivity toward electrophilic addition (an electronegativity of sp-hybridized carbon atom higher than an electronegativity of sp2-hybridized carbon atom.
In the presence of mercuric sulfate catalyst, however, hydration occurs readily occurs with Marcovnikov regiochemistry:
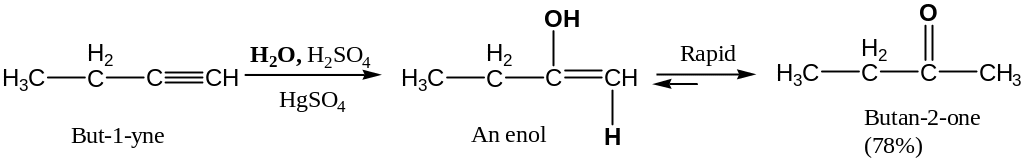
The individual keto and enol forms are tautomeres (constitutionale isomers that are rapidly interconverted.
Keto tautomer is more favored than enol tautomer.
Hydroboratin/Oxidation of alkynes
Borane adds rapidly to alkynes. Internal alkynes give vinylic borane; relatively unhindered terminal alkyne undergoes two additions, giving a doubly hydroborated intermediate.
Oxidation with H2O2 at pH 8 then replaces boron atoms by oxygen and generates the ketone or aldehyde, depending on the structure of the alkyne reactant.
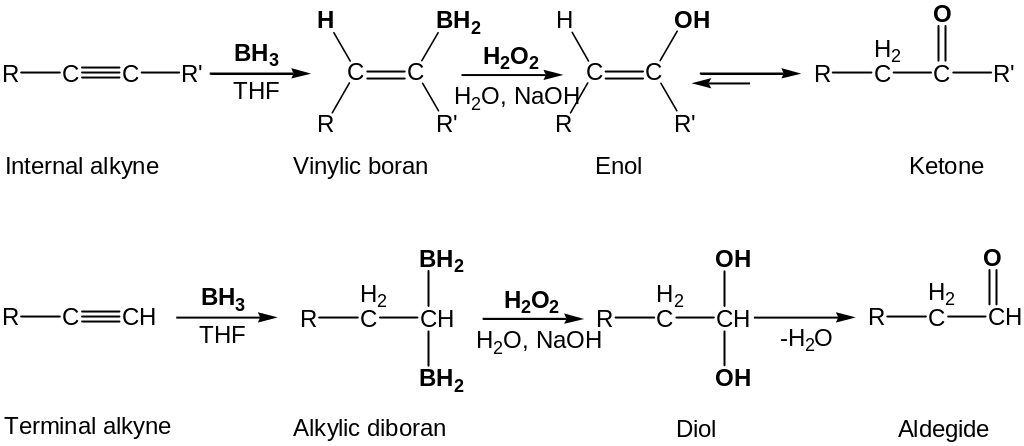
The hydroboratin/oxidation sequence is complementary to the direct Kutcherov’s reaction because different products result.

Reduction of alkynes
The reaction over a metal katalyst occurs in steps through an alkane intermediate.
First step in the reaction has larger ∆H0 than the second step

As a result, alkynes reduce somewhat more readily than alkenes.
With using different catalyst different reduction products can be preparated.

Pd/C is palladium on carbom
Li/NH3 is litium (or sodium) metal in liquid ammonia as solvent.
Oxidative cleavage of alkynes
Alcynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or KMnO4.
The products obtained from cleavage of an internnal alkyne are carboxylic acids; from a terminal alkyne, CO2 is formed as one product

Alkynes oxidation reaction is used in alkynes structure determination for establishment of triple bond position.
Alkyne Acidity: Formation of Acetylide Anions
When a terminal alkyne is treated with a strong base, such as sodium amide, NaNH2, the terminal hydrogen is removed and an acetylide anion is formed

Since a terminal alkyne (pKa ~25) is a stronger acid than ammonia, NH3, (pKa =35), amide ion (NH2-), the anion of ammonia, removes proton from terminal alkyne.
Acidity of Simple Hydrocarbons
Tipe |
Example |
Ka |
pKa |
|
Alkyne
Alkene
Alkane |
CH≡CH
CH=CH
CH–CH |
10–25
10–44
~10–60
|
25
44
~ 60 |
Stronger acid
Weaker acid |
Terminal akkynes are more acidic than alkenes or alkanes
An acid is stronger when anion of conjugate base is more stable.

Since s orbitals are lower in energy and nearer the positively charged nucleus than are p orbitals, an negative charge is stabilized to a greater extent in an orbital with high s character than in an orbital with low s character.
Acetylide anions are therefore more stable than vinylic anions, which, in turn, are more stable than alkyl anions.
Alkylation of Acetylide Anions
A reaction is called an alkylation
because a new alkyl group has become attached to the starting
compound.
reaction is called an alkylation
because a new alkyl group has become attached to the starting
compound.
The presence of a negative charge and an unshared electron pair on carbon makes acetylide anions strongly nucleophilic.
As a result, acetylide anions can react with alkyl halides and yield a new alkyne product.
Alkyne alkylation is not limited to acetylide ion. Any terminal alkyne can be converted into its corresponding anion and then alkylated by treatment with an alkyl halide. The product is an internal alkyne.
For example:
![]()
Acetylide ion alkylation is limited to the use of primary alkyl bromides and iodides, RCH2X.
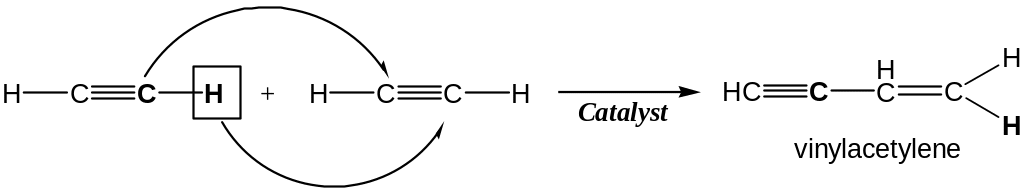
Dimerization of Alkynes
Dimerization of acetylene to vinylacetylen occurs when CuCl and NH4Cl is used as catalyst
Trimerization of Alkynes
Trimerization of alkyne to the benzene or alkylbenzenes occurs when complex nikelorganic compound, as Ni(CO)2[(C6H5)3P]2, is used as catalyst

Tetramerization of Alkynes
Tetramerization of acetylene to the cyclooktatetraene occurs when Ni(CN)2 is used as catalyst


