

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
1. AIM OF THE WORK:
Skills studying of the laws of thermal radiation.
TASKS:
definition of the Stefan-Boltzmann constant, knowledge of the work of an optical pyrometer.
2. INTRODUCTION
2.1 Thermal radiation of the bodies
The electromagnetic radiation emitted by the atoms of the body due to the internal (thermal) energy of the radiating body and depended only on the temperature and the optical properties of the body is called thermal radiation. It occurs because of the thermal motion of the particles of the body and its characteristics: the intensity and spectral composition - are dependent on the temperature of the body. Thermal electromagnetic radiation occurs at all frequencies, but with varying intensity.
This type of radiation happens at all temperatures and is of particular interest to physicists because it is the only radiation that can be in thermal equilibrium with heated objects, i.e., the distribution of energy between the body and the radiation field remains the same for any frequency of radiation.
Heated bodies change by energy only by the emission and absorption of radiant energy. In equilibrium, the emission and absorption processes of each body on average compensate each other, and in the space between the bodies the radiation characteristics reach a certain values, depending only on the established bodies
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
temperature. This radiation, which is in thermodynamic equilibrium with the bodies having the same temperature, is called the equilibrium radiation or black radiation. The amount of energy of the equilibrium radiation and its spectral distribution depend only on the temperature. If in adiabatically closed cavity with a mirror reflecting walls to put some bodies heated to different temperatures, then, as the experiment shows, such a system over time goes into a state of thermal equilibrium, where all of the bodies have the same temperature. If you look through a small hole into the cavity in which the thermodynamic equilibrium is established between the radiation and heated objects, the eye cannot distinguish the outlines of bodies and fixes a uniform illumination of the whole cavity in general.
To establish the equilibrium in the cavity is necessary that everybody emits as much radiant energy as it absorbs. This is one of the most important principles of thermal radiation, experimentally established by Prevost in 1809.
2.1 Laws of thermal radiation
To understand the laws of thermal radiation consider the used values characterizing the thermal radiation.
Energy luminosity of the body Rl is the amount of electromagnetic energy emitted in all directions of the unit body surface in time unit. Energy luminosity is the function of temperature.
Radiation consists of waves of different frequencies. Denote the flow of energy
emitted per unit area of the body in the frequency range from to |
+ |
through |
|
. If the interval |
is small, then |
|
|
|
. (1) |
|
|
The value |
is the spectral characteristic of the emission. |
It |
is energy |
luminosity, related to the frequency unit interval near a given frequency ν, and is called the emissivity of the body. The connection between the energy luminosity and the emissivity of the body is expressed as
∫ . (2)
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
All bodies in varying degrees absorb energy of incident electromagnetic waves.
Absorptivity of the body аvT is the spectral absorption characteristics. It shows how much of light energy, falling on the surface of the body that contains electromagnetic waves with frequencies ranging from to + is absorbed by the body. The body, completely absorbing the incident on it radiation of all frequencies, is an absolutely black. For it аvT=1. For all other bodies аvT <1. Close to absolute black bodies one can consider, for example, carbon black, platinum black.
On the basis of experimental data Stephan and Boltzmann found that energy luminosity of a black body R increases proportionally of fourth power of the absolute temperature of the body
, (3)
where σ = 5.6703 ×10−8W/m2K4 is the Stefan’s- Boltzmann constant.
The connection between the emissivity and absorptivity of any body is described by the Kirchhoff law
( ) ( ) ( ) , (4)
where 1,2,…n indexes characterized the different bodies. From the equation (4) it follows, that the ratio of the emissivity and absorptivity doesn’t depend on the nature
of the body. It is the same founction |
for all bodies, depending on the frequency |
|
(or wave length , since |
) and temperature T. It is easy to see that the |
|
physical sense of the universal Kirchhoff’s function |
is nothing else than the |
|
emissivity of a black body. |
|
|
Experimentally established the Kirchhoff type of function at different temperatures is shown in figure 1 (a, b). In particular from the figure it is shown that the increase in
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
temperature leads to a shift in the wavelength λm, which is a maximum emissivity of the black body, to shorter wavelengths. Wien's displacement law states that λm varies inversely proportional to the temperature:
(5)
where b is the Wien's constant, |
. |
a b
Figure 1 - The spectral distribution of black-body radiation at different temperatures
Wien established and the second law, according to which the maximum value fo the emissivity of a black body rm increases direct proportionally to the fifth power of the temperature:
|
, (6) |
|
where |
. |
|
Attempts to obtain a universal Kirchhoff’s function |
in the thermodynamic |
|
approach failed. Rayleigh and Jeans, using the classical Boltzmann law about the
PhysicVirtualLabs Project Team, IITU
Copyright 2014
LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
uniform energy distribution on the degrees of freedom, got the equation for the
Kirchhoff’s function:
|
|
|
|
|
|
, (7) |
|
|
|
|
|
|
|
where |
is the average energy oscillation, |
making the oscillations with |
||||
fundamental frequency , k is the Boltzmann constant, |
. |
|||||
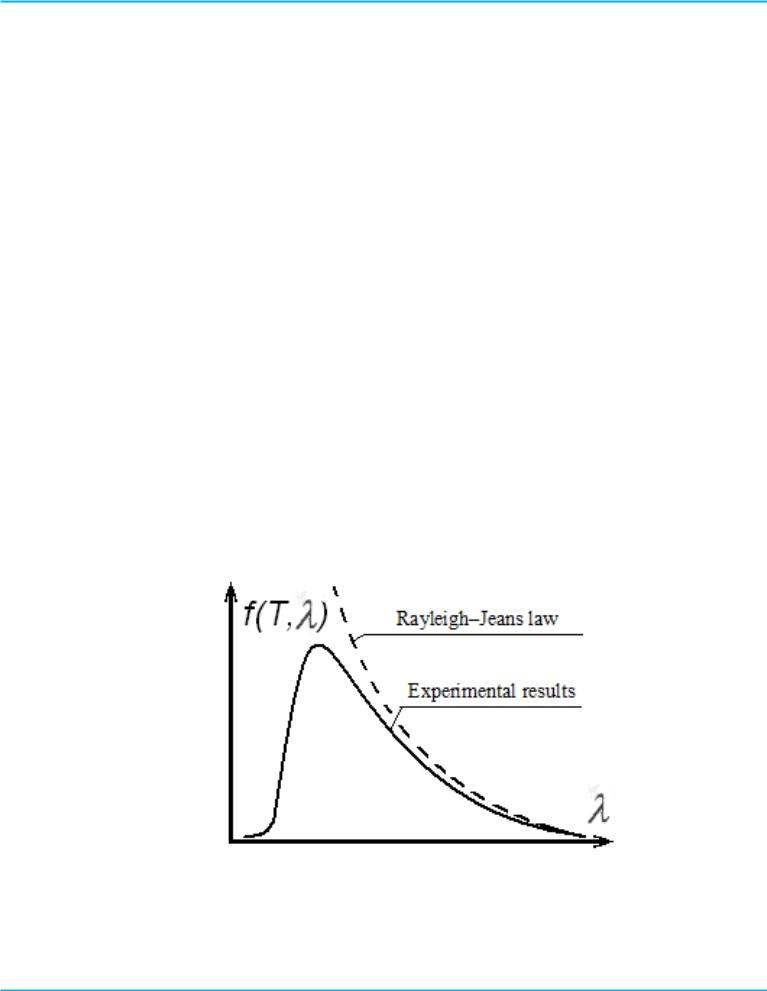
Experience has shown that the Rayleigh-Jeans equation (7) is in a satisfactory agreement with the experimental data only at long wavelengths (or low frequencies) and high temperatures (Figure 2). Furthermore, according to the Rayleigh-Jeans the energy luminosity Rl of a blackbody in the ultraviolet range wavelengths is infinitely great, that in general has no physical meaning. This result is called "ultraviolet catastrophe" (This “catastrophe” - infinite energy - occurs as the wavelength approaches zero; the word ultraviolet was applied because ultraviolet wavelengths are short.) The divergence between the Rayleigh-Jeans equation with the experimental data led to the conclusion that there are such regularities of the thermal radiation, which are inconsistent with the fundamental positions of classical statistical physics and electrodynamics.
Figure 2 - Comparison of experimental results and the curve predicted by the Rayleigh–Jeans law for the distribution of blackbody radiation
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
Only in 1900, Planck found the form of the Kirchhoff’s function precisely corresponding to the experimental data over the entire range of frequencies. To do this, he had to refuse from the established position of classical physics, according to which the energy of any system can vary continuously i.e. it can take the arbitrarily close values. Plank proposed the quantum hypothesis according to which the atomic oscillators emit the energy not continuously, but in certain portions - quantum, and the energy of quantum ε is proportional to the frequency of the radiation
, (8) |
|
|
|
|
||||||||||
where h is the universal constant, called the Planck constant, |
. |
|||||||||||||
Planck shown that at this condition the average energy |
|
|
of oscillator is equal to: |
|||||||||||
|
|
|
|
|
|
|
|
, (9) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
and the Kirchhoff’s function has the view: |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
. (10) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
One can say that taking into account |
and equation (10) the |
function |
||||||||||||
will have the view: |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
. (11) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equations (10) and (11) are the Planck’s equations. From them, as a result, may be obtained by the Stefan-Boltzmann, Wien and Rayleigh-Jeans laws, and the StefanBoltzmann constant σ and Wien constant b can be calculated [1, 2].
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
Brilliant agreement Planck equation with experimental data proves the correctness of the assumptions made by him about the discreteness of radiation energy of the atomic oscillator and the equation (8).
Using the Planck’s equation (10) and the Kirchhoff’s law (4) one can determine the emissivity of any real body:
|
|
|
. (12) |
For all |
natural bodies |
, hence |
. The function , usually |
differs from |
. The exception is the so-called gray bodies, for which the value |
||
remains constant in wide frequency range. The energy distribution in the spectrum of a gray body is the same as that of a black, but the magnitude of the radiation energy is less.
3. EXPERIMENTS DESCRIPTION
Task 1:
In this task will be defined the Stefan’s constant.
The source of the thermal radiation is tungsten glow-lamp filament (no black body), that’s way for calculations is used the equation:
|
(13) |
For this case emissivity is |
. Glow-lamp is connected to the AC circuit as |
it is shown in the Figure 1. |
|
Changing a current I using the resistance R1 in lamp, it is possible to get different degree of heating (temperature) of the source. Radiation power of the lamp is calculated by well-known equation P=IU, where I and U are defined from the amperemeter A1 and voltmeter V1 .
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
Figure 1 - AC circuit
Suggesting that all emitted power spends to the thermal radiation, we can express radiation power R by the following form:
, (14)
where S is common surface of the red-hot part of lamp filament, its magnitude in this work is .
Substituting the value of R from formula (14) to the left side of the expression
(13) and solving the obtained equation relative to σ, we get working equation:
. (15)
Thus, calculation of the Stefan’s constant σ is related to measurements of current
I, voltage U on the lamp and temperature of glow-lamp filament T. Т0 can be taken as temperature of the room, measured by thermometer.
Task 2:
PhysicVirtualLabs Project Team, IITU
Copyright 2014

LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
In this work we use contactless optical pyrometer. The Optical Pyrometer is a highly-developed and well accepted noncontact temperature measurement device with a long and varied past from its origins more than 100 years ago. In spite of the fact that more modern, automatic devices have nearly displaced it, several makers still produce and sell profitable quantities each year. In Figure 2 optical pyrometer is presented. In this virtual laboratory work optical pyrometer ЭОП-66 was modeled to measure brightness temperature (in range from 1073K to10273 K) of hot objects by theirs thermal radiation in visible part of spectrum. We use temperature range 1400-
2000°C which fit to temperature from used radiation source in certain range of voltage.
Pyrometer can measure temperature of the source at the distance from 0.7m to infinity.
Figure 2 - Optical pyrometer
In Figure 3 you can see scheme of the optical pyrometer. Optical Pyrometers work on the basic principle of using the human eye to match the brightness of the hot object to the brightness of a calibrated lamp filament inside the instrument. The optical system contains filters that restrict the wavelength-sensitivity of the devices to
PhysicVirtualLabs Project Team, IITU
Copyright 2014
LABORATORY WORK #5
DEFINITION OF STEFAN-BOLTZMANN CONSTANT
PhysicVirtualLab Software Package
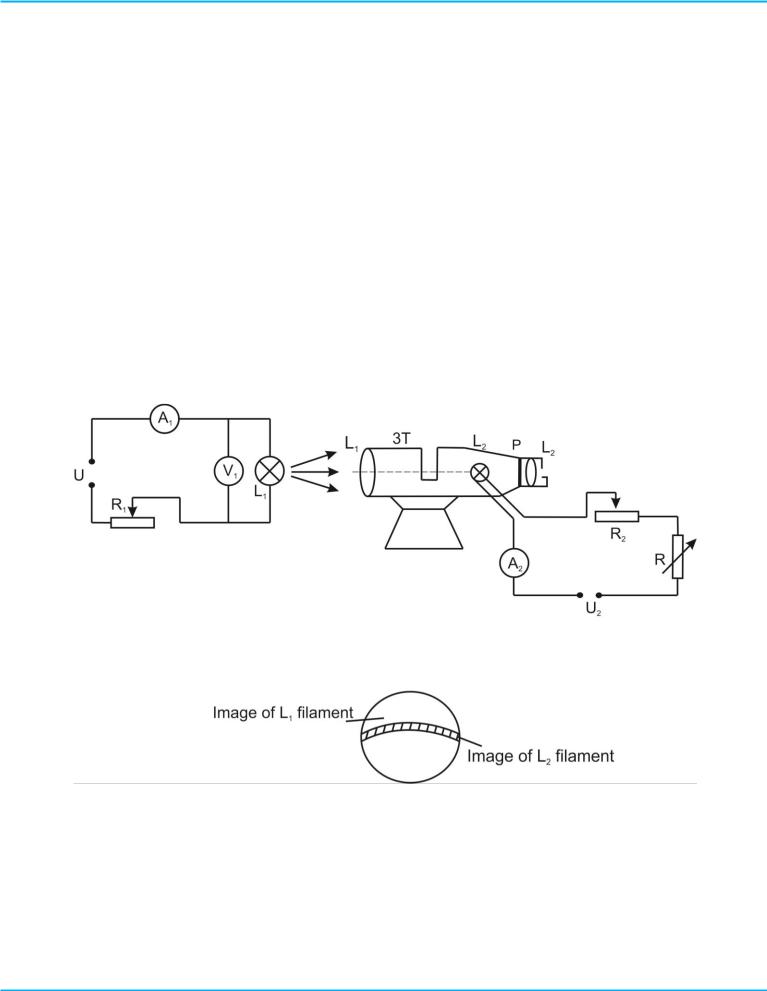
a narrow wavelength band around 0.65 to 0.66 microns (the red region of the visible spectrum). Optical pyrometer consists of telescope ЗТ at the focal point of lens L1 where filament of standard lamp L2 is located. Image of the radiating source is projected on the filament surface of lamp L2.
Observer should measure the temperature of radiating source. Observer, who is at the lens L2 , can see the filament of lamp L2 like arched (curved) light on the background image of the radiating source, which is limited by diaphragm Д (Figure 3, b). Glow filament of lamp L2 can be changed by rheostat R2. By changing of the current in lamp, its brightness can be equalized with brightness of the radiating source. In this case working part of the filament is disappeared; it means that temperatures of filaments for lamp L2 and for radiating source are the same. By measuring of corresponding current of lamp L2 due to the ammeter А2, it is possible to define the temperature of the object using the table or the calibration curve (Figure 4).
a
b
Figure 3 - Scheme of the optical pyrometer
PhysicVirtualLabs Project Team, IITU
Copyright 2014
