
Meyer R., Koehler J., Homburg A. Explosives. Wiley-VCH, 2002 / Explosives 5th ed by Koehler, Meyer, and Homburg (2002)
.pdf
147 |
Fuse |
|
|
nitrous gases; the sampling must take place within a period of 1 to 9 minutes after the firing. Glass test tubes of 500 or 1000 ml capacity, provided with a faucet on either side, should be used for the sampling. Prior to the firing, 20 ml of 0.1 n potassium hydroxide should be filled in the test tube intended for the nitrous gas analysis. The residual air pressure present in each of the test tubes should be measured for the subsequent calculation. The carbon monoxide content should be determined by the Wösthoff conductivity method to or by infrared absorption. The nitrous gas content should be determined approx. 24 hours after sampling, applying the method of photometrical evaluation of the color reaction according to Gries/v. Ilosvay. Duplicate analyses should be made for every test tube. From the resulting 2 V 9 individual values, the mean should be determined. The mean values have to be stated in litres per kilogram of detonated explosive. In addition, the following test conditions have to be indicated: type and quality of the rock, number and diameter of boreholes, average length of borehole, total charge quantity, borehole spacings and burden, mode of ignition and stemming.
An explosive is considered harmless if the first test produces a maximum of 32 l carbon monoxide and a maximum of 4.0 l nitrous gases per kg of explosive. If the result for carbon monoxide exceeds 32, but not 40 l per kg of explosive or the result for nitrous gases is over 4.0 but not over 5.0 l per kg of explosive, the fume test must be repeated. The mean values for carbon monoxide and nitrous gases from the results of the first and the second test must not exceed 40 l of carbon monoxide per kg of explosive and 5.0 l of nitrous gases per kg of explosive.
Functioning Time*)
Anzündverzugszeit; retard d’allumage
Lapsed time between application of firing current to start of pressure rise.
Fuse*)
An igniting or explosive device in form of a cord, consisting of a flexible fabric tube and a core of low or high explosive. Used in blasting and demolition work, and in certain munitions. A fuse with a black powder or other low explosive core is called a safety fuse or blasting fuse. A fuse with a W PETN or other high explosive core is called “detonating cord” or primacord.
* Text quoted from glossary.

Fuze |
148 |
|
|
Fuze*)
Zünder, Anzünder; fus´ee
A device with explosive or pyrotechnic components designed to initiate a train of fire or detonation.
Fuze, delay. Any fuze incorporating a means of delaying its action. Delay fuzes are classified according to the length of time of the delay.
Fuze, long delay. A type of delay fuze in which the fuze action is delayed for a relatively long period of time, depending upon the type, from minutes to days.
Fuze, medium delay. A type of delay fuze in which the fuze action is delayed for a period of time between that of short delay and long delay fuzes, normally four to fifteen seconds.
Fuze Head*)
Zündschraube, Anzündschraube
A device for the ignition of a gun propellant. It consists of a percussion cap, containing a small amount of black powder booster in front, and a threaded armature part screwed into the base of the cartridge.
Gap Test
W Detonation, Sympathetic Detonation; Flash Over.
Gas Generators*)
gaserzeugende Ladungen; charges g´en´eratrices de gaz
Pyrotechnic or propellant device in which propellant is burned to produce a sustained flow of gas at a given pressure on demand. Gasgenerating units are employed in blasting operations conducted in mines without recourse to brisant explosives. The device consists of a non-detonating gas-generating material and a priming or a heating charge, which are confined together in a steel pipe. The heating charge evaporates the gas-generating substance such as liquid CO2 (W Cardox); another possibility is for the primer to initiate an exothermal chemical reaction (Chemecol process, Hydrox process). The gas-generating reaction may be the decomposition of nitrogen-rich compounds such as ammonium nitrate, or nitrate mixtures, or nitroguanidine in the presence of carbon carriers and sometimes in the
*Text quoted from glossary. The spelling and notion “fuze” is common in the USA.

, Fifth Edition Rudolf Meyer, Josef Köhler, Axel Homburg
Fuze*)
Zünder, Anzünder; fus´ee
A device with explosive or pyrotechnic components designed to initiate a train of fire or detonation.
Fuze, delay. Any fuze incorporating a means of delaying its action. Delay fuzes are classified according to the length of time of the delay.
Fuze, long delay. A type of delay fuze in which the fuze action is delayed for a relatively long period of time, depending upon the type, from minutes to days.
Fuze, medium delay. A type of delay fuze in which the fuze action is delayed for a period of time between that of short delay and long delay fuzes, normally four to fifteen seconds.
Fuze Head*)
Zündschraube, Anzündschraube
A device for the ignition of a gun propellant. It consists of a percussion cap, containing a small amount of black powder booster in front, and a threaded armature part screwed into the base of the cartridge.
Gap Test
W Detonation, Sympathetic Detonation; Flash Over.
Gas Generators*)
gaserzeugende Ladungen; charges g´en´eratrices de gaz
Pyrotechnic or propellant device in which propellant is burned to produce a sustained flow of gas at a given pressure on demand. Gasgenerating units are employed in blasting operations conducted in mines without recourse to brisant explosives. The device consists of a non-detonating gas-generating material and a priming or a heating charge, which are confined together in a steel pipe. The heating charge evaporates the gas-generating substance such as liquid CO2 (W Cardox); another possibility is for the primer to initiate an exothermal chemical reaction (Chemecol process, Hydrox process). The gas-generating reaction may be the decomposition of nitrogen-rich compounds such as ammonium nitrate, or nitrate mixtures, or nitroguanidine in the presence of carbon carriers and sometimes in the
*Text quoted from glossary. The spelling and notion “fuze” is common in the USA.
149 |
Gas Pressure |
|
|
presence of catalysts. When a given pressure has been reached, a bursting disc releases the gases in the pipe. The sudden gas expansion taking place in the borehole has an effect similar to that of an explosion.
Gas Jet Velocity
Ausströmgeschwindigkeit; v´elocit´ a` jet de gaz
In rocket technology, the velocity of the combustion gases discharged from the combustion chamber and passing the nozzle into the atmosphere. The jet velocity and the mass flow serve to calculate the W Thrust. The jet velocity will increase with the pressure in the combustion chamber, i.e., with the expansion ratio under passage through the W Nozzle. The pressure in the combustion chamber should not be adjusted too high, otherwise the wall thickness of the chamber (i.e. its weight) will become too great (W Mass Ratio).
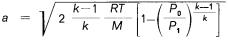
In accordance with the Saint-Venant and Wantzel formula
where:
P0 is the gas pressure at the nozzle exit (atmospheric pressure); P1 is the pressure in the combustion chamber;
k is the coefficient of specific heat;
R is the ideal gas constant measured in absolute units; T is the flame temperature in Kelvin, and
M is the mean molecular weight of the combustion gases.
The jet velocity is proportional to the square root of the combustion temperature and inversely proportional to the square root of the mean molecular weight of the combustion gases.
Other details can be deduced from the formula.
Other keywords in this connection: W Propellant Area Ratio W Solid Propellant Rockets.
Gas Pressure
Gasdruck; pression de gaz
The pressure generated in the chamber of a weapon; its value depends to a large extent on the nature of the weapon and of the powder selected. Standard determinations of gas pressure are carried out with the aid of a crusher (“measuring egg”) – a copper cylinder or a copper

Gelatine Donarit 1; 2; 2 E; S |
150 |
|
|
pyramid – the compression of which is a measure of the gas pressure.
A complete gas pressure curve can only be plotted with the aid of piezo-quartz or other pressure recorder in conjunction with an oscillograph (W Ballistic Bomb).
Gelatine Donarit 1; 2; 2 E; S
Trade names of gelatinous explosives containing ammonium nitrate distributed in Austria by DYNAMIT NOBEL WIEN.
Gelatine |
Density |
Weight |
Detonation Velocity |
|
Donarit |
g/cm3 |
Strength |
confined: |
|
|
% |
m/s |
ft/s |
|
|
|
|
|
|
1 |
1.5 |
85 |
6000 |
19 700 |
2 |
1.5 |
87 |
6200 |
20 300 |
2 E |
1.5 |
84 |
6000 |
19 700 |
S |
1.6 |
80 |
6500 |
21 300 |
|
|
|
|
|
Gelatine Donarit 2 E and S are explosives for special purposes.
2 E for smooth blasting
S for seismic prospecting
Gelatins; Gelatinous Explosives; Gelignites
They are being replaced by cartridged
WEmulsion Slurries.
WPlastic Explosives, on the other hand, are dough-like mixtures of highly brisant crystalline explosives (e.g. RDX, W Cyclonite) with plasticizing agents, W Plastic Explosives.
Geosit 3
Trade name of a sensitized gelatinous special explosive distributed in Germany and exported by WASAGCHEMIE. It is used for seismic prospecting and mud capping.
The explosive can be supplied as a cartridge in sealable plastic tubes.
density of cartridge: |
1.6 g/cm3 |
weight strength: |
81 % |

151 |
Glycerol Chloride Dinitrate |
|
|
detonation velocity at cartridge density,
unconfined: 6100 m/s
= 20 000 ft/s
GGVE
Gefahrgutverordnung, Eisenbahn
German transport regulation; W RID
Glycerol Acetate Dinitrate
Acetyldinitroglycerin; ac´etate-dinitrate de glyc´erine
pale yellow oil
empirical formula: C5H8N2O8 molecular weight: 224.1 oxygen balance: – 42.86 % nitrogen content: 12.50 % density: 1.412 g/cm3
lead block test: 200 cm3/10 g
deflagration point: 170 –180 °C = 338 – 356°F
This compound is insoluble in water, but is readily soluble in alcohol, ether, acetone, and concentrated HNO3.
It may be prepared by nitration of acetylglycerol with mixed acid containing a very large proportion of nitric acid.
Glycerol acetate dinitrate has been proposed as an additive to nitroglycerine in order to depress the solidification point of the latter. It has so far not been employed in practice.
Glycerol Chloride Dinitrate
Dinitrochlorhydrin; dinitrate-chlorure de glyc´erine
pale yellow liquid
empirical formula: C3H5N2O6Cl
Glycerol Dinitrate |
152 |
|
|
molecular weight: 200.5 oxygen balance: –15.9 % nitrogen content: 13.97 % density: 1.54 g/cm3
solidification point: +5 °C = 41°F lead block test: 475 cm3/10 g detonation velocity, confined:
6750 m/s = 22 100 ft/s at r = 1.54 g/cm3 deflagration point: 190 °C = 393°F
impact sensitivity: 0.7 kp m = 7 N m
Dinitrochlorohydrin is not hygroscopic and is practically insoluble in water. It is more volatile and less viscous than nitroglycerine.
Glycerol Dinitrate
Dinitroglycerin, Glycerindinitrat; dinitrate de glyc´erine
pale yellow oil
empirical formula: C3H6N2O7 molecular weight: 182.1 oxygen balance: –17.6 % nitrogen content: 15.38 % density: 1.51 g/cm3
solidification point: – 30 °C = – 22°F lead block test: 450 cm3/10 g deflagration point: 170 °C = 338°F impact sensitivity: 0.15 kp m = 1.5 N m
Glycerol dinitrate is a viscous liquid, but is more volatile and more soluble in water than nitroglycerine. It is hygroscopic and may be used as a gelatinizer of certain types of nitrocelluloses. It is more stable than glycerol trinitrate. Its vapors are toxic and cause headaches.
It is prepared by nitration of glycerol with nitric acid; such nitrations mostly yield mixtures of diand trinitroglycerine.

153 |
Glycerol Nitrolactate Dinitrate |
|
|
Glycerol – 2,4-Dinitrophenyl Ether Dinitrate
Dinitrophenylglycerinätherdinitrat;
dinitrate de glyc´erine-dinitroph´enyl´ether, Dinitryl
pale yellow crystals empirical formula: C9H8N4O11 molecular weight: 348.2 oxygen balance: – 50.6 % nitrogen content: 16.09 % density: 1.60 g/cm3
melting point: 124 °C = 255°F lead block test: 320 cm3/10 g deflagration point: 205 °C = 400°F
impact sensitivity: 0.8 kp m = 8 N m
This compound is prepared by reacting glycerol nitrophenyl ether with a nitric acid – sulfuric acid mixture at 25 – 30 °C (~77 °F). It is insoluble in water, but is readily soluble in acetone. It is a poor gelatinizer of nitrocellulose.
Glycerol Nitrolactate Dinitrate
Dinitroglycerinnitrolactat; dinitrate-nitrolactate de glyc´erine
colorless liquid
empirical formula: C6H9N3O11 molecular weight: 299.2 oxygen balance: – 29.7 % nitrogen content: 14.05 % density: 1.47 g/cm3
refractive index: n25D = 1.464 deflagration point: 190 °C = 374°F
Dinitroglycerol nitrolactate is practically insoluble in water, readily soluble in alcohol and ether, and is a good gelatinizer of nitrocellulose. It is more resistant to heat and less sensitive to impact than nitroglycerine.

Glycerol Trinitrophenyl Ether Dinitrate |
154 |
|
|
Glycerol Trinitrophenyl Ether Dinitrate
Trinitrophenylglycerinätherdinitrat; dinitrate de trinitrophenyl-glyc´erine´ether
yellowish, light-sensitive crystals empirical formula: C9H7N5O13 molecular weight: 393.2
oxygen balance: – 34.6 % nitrogen content: 17.81 %
solidification point: 128.5 °C = 263.3°F lead block test: 420 cm3/10 g
deflagration point: 200 – 205 °C = 392 – 400°F impact sensitivity: 0.4 kp m = 4 N m
Glycerol trinitrophenyl ether dinitrate is insoluble in water, but is readily soluble in acetone; it does not gelatinize nitrocellulose.
It is prepared by nitration of phenyl glycerol ether with a nitric acidsulfuric acid mixture.
Glycidyl Azide Polymer
Glycidylazidpolymer; GAP
light-yellowish, viscous liquid
empirical formula of structural unit: C3H5N3O molecular weight of structural unit: 99.1 mean molecular weight: 2000
energy of formation: +1535.2 kJ/kg = +366.9 kcal/kg enthalpy of formation: +1422.9 kJ/kg = +340.1 kcal/kg oxygen value: –121.1 %
nitrogen content: 42.40 %
specific energy: 82.4 mt/kg = 808 kJ/kg
explosion heat (H2O liq.): 3429 kJ/kg = 820 kcal/kg normal volume of gases: 946 l/kg
viscosity: 4280 cP density: 1.3 g/cm3
deflagration temperature: 216 °C

155 |
Graphite |
|
|
impact sensitivity: 7.9 Nm = 0.8 kpm
sensitivity to friction: at 360 N = 37 kp pin load, no reaction
Glycidyl azide polymer is produced in a two-step process. First, epichlorhydrine in the presence of bortriflouride, is polymerized into polyepichlorhydrine. Using dimethylformamide as a solvent, the polymer is then processed with sodium azide at high temperature. Nearly all the inorganic components as well as the solvent are removed, leaving the raw final product free of low molecular weight compounds.
Glycidyl azide polymer was originally developed in the USA as an
W Active Binder for W Composite Propellants. Because this gas-pro- ducing component has been shown to have a low explosion temperature, it has been used in recent years as an active binder compound in W LOVA gun propellant.
Grain*)
A single mass of solid propellant of the final geometric configuration as used in a gas generator or rocket motor.
Granulation*)
Size and shape of grains of pyrotechnic or propellant ingredients (W Grist)
Graphite
C
atomic weight: 12.01
serves for surface smoothing of flake-grained W Gunpowder and of
W Black Powder.
Specifications |
|
moisture: not more than |
0.5 % |
reaction: |
neutral |
glow residue in natural graphite: |
|
not more than |
25 % |
* Text quoted from glossary.
