
26510318-Bio-Transformation-of-Xenobiotics
.pdf
CHAPTER 6
BIOTRANSFORMATION OF
XENOBIOTICS
Andrew Parkinson
GENERAL PRINCIPLES
Basic Properties of Xenobiotic Biotransforming Enzymes
Biotransformation versus Metabolism Stereochemical Aspects of Xenobiotic
Biotransformation
Phase I and Phase II Biotransformation Nomenclature of Xenobiotic Biotransforming
Enzymes
Distribution of Xenobiotic Biotransforming Enzymes
XENOBIOTIC BIOTRANSFORMATION BY PHASE I ENZYMES
Hydrolysis
Carboxylesterases, Pseudocholinesterase, and
Paraoxonase
Peptidases
Epoxide Hydrolase
Reduction
Azoand Nitro-Reduction
Carbonyl Reduction
Disulfide Reduction
Sulfoxide and N-Oxide Reduction
Quinone Reduction
Dihydropyrimidine Dehydrogenase
Dehalogenation
Oxidation
Alcohol, Aldehyde, Ketone Oxidation-Reduction
Systems
Alcohol Dehydrogenase
Aldehyde Dehydrogenase Dihydrodiol Dehydrogenase
Molybdenum Hydroxylases (Molybdozymes) Xanthine Dehydrogenase–Xanthine Oxidase Aldehyde Oxidase
Monoamine Oxidase, Diamine Oxidase, and Polyamine Oxidase
Aromatization Peroxidase-Dependent Cooxidation Flavin Monooxygenases Cytochrome P450
Activation of Xenobiotics by Cytochrome P450 P450 Knockout Mice
Inhibition of Cytochrome P450
Induction of Cytochrome P450
PHASE II ENZYME REACTIONS
Glucuronidation
Sulfation
Methylation
Acetylation
Amino Acid Conjugation
Glutathione Conjugation
Rhodanese
Phosphorylation—The Dog That Did Not Bark
All organisms are exposed constantly and unavoidably to foreign chemicals, or xenobiotics, which include both manufactured and natural chemicals such as drugs, industrial chemicals, pesticides, pollutants, pyrolysis products in cooked food, alkaloids, secondary plant metabolites, and toxins produced by molds, plants, and animals. The physical property that enables many xenobiotics to be absorbed through the skin, lungs, or gastrointestinal tract —namely, their lipophilicity—is an obstacle to their elimination, because lipophilic compounds can be readily reabsorbed. Consequently, the elimination of xenobiotics often depends on their conversion to wa- ter-soluble chemicals by a process known as biotransformation, which is catalyzed by enzymes in the liver and other tissues. An important consequence of biotransformation is that the physical properties of a xenobiotic are generally changed from those favoring absorption (lipophilicity) to those favoring excretion in urine or feces (hydrophilicity). An exception to this general rule is the elimination of volatile compounds by exhalation, in which case
biotransformation to nonvolatile, water-soluble chemicals can retard their rate of elimination. Similarly, biotransformation of xenobiotics in the brain and testis (two organs with a barrier to chemical transport) might also be an obstacle to xenobiotic elimination if the metabolites cannot cross the blood-brain or blood-testis barrier.
Without biotransformation, lipophilic xenobiotics would be excreted from the body so slowly that they would eventually overwhelm and kill an organism. This principle is illustrated by comparing the theoretical and observed rates of elimination of barbital and hexobarbital. The theoretical half-life of barbital, which is water-soluble, closely matches the observed elimination half-life of this drug (t1 2 55 to 75 h), which is eliminated largely unchanged. In contrast, the observed elimination half-life of hexobarbital (t1 2 5 to 6 h) is considerably shorter than the theoretical rate of elimination of this highly lipophilic drug (t1 2 2 to 5 months), the difference being that hexobarbital is biotransformed to watersoluble metabolites that are readily excreted.
133

134 |
UNIT 2 DISPOSITION OF TOXICANTS |
A change in pharmacokinetic behavior is not the only consequence of xenobiotic biotransformation nor, in some cases, is it the most important outcome. Xenobiotics exert a variety of effects on biological systems. These may be beneficial, in the case of drugs, or deleterious, in the case of poisons. These effects are dependent on the physicochemical properties of the xenobiotic. In many instances, chemical modification of a xenobiotic by biotransformation alters its biological effects. The importance of this principle to pharmacology is that some drugs must undergo biotransformation to exert their pharmacodynamic effect (i.e., it is the metabolite of the drug, and not the drug itself, that exerts the pharmacologic effect). The importance of this principle to toxicology is that many xenobiotics must undergo biotransformation to exert their characteristic toxic or tumorigenic effect (i.e., many chemicals would be considerably less toxic or tumorigenic if they were not converted to reactive metabolites by xenobiotic-biotransforming enzymes). In most cases, however, biotransformation terminates the pharmacologic effects of a drug and lessens the toxicity of xenobiotics. Enzymes catalyzing biotransformation reactions often determine the intensity and duration of action of drugs and play a key role in chemical toxicity and chemical tumorigenesis (Anders, 1985; Jakoby, 1980; Jakoby et al., 1982; Kato et al., 1989).
To a limited extent, the degree to which organisms are exposed to xenobiotics determines their biotransformation capacity. For example, insects that feed on a variety of plants have a greater capacity to biotransform xenobiotics than insects that feed on a limited number of plants; these, in turn, have a greater capacity to biotransform xenobiotics than insects that feed on a single species of plant. Compared with mammals, fish have a low capacity to metabolize xenobiotics, ostensibly because they can eliminate xenobiotics unchanged across their gills. Species differences in the capacity of mammals to biotransform xenobiotics do not simply reflect differences in exposure. However, some chemicals stimulate the synthesis of enzymes involved in xenobiotic biotransformation. This process, known as enzyme induction, is an adaptive and reversible response to xenobiotic exposure. Enzyme induction enables some xenobiotics to accelerate their own biotransformation and elimination (Conney, 1967). The mechanism and consequences of enzyme induction are discussed further on, under “Induction of Cytochrome P450.”
thesis of steroid hormones are catalyzed by cytochrome P450 enzymes in steroidogenic tissues. In general, these steroidogenic enzymes play little or no role in the biotransformation of xenobiotics. In the liver, however, other cytochrome P450 enzymes convert steroid hormones to water-soluble metabolites that are excreted in urine or bile, in an analogous manner to the biotransformation and elimination of xenobiotics.
The structure (i.e., amino acid sequence) of a given biotransforming enzyme may differ among individuals, which can give rise to differences in rates of xenobiotic biotransformation. In general, a variant form of a xenobiotic biotransforming enzyme (known as an allelic variant or an allelozyme) has diminished enzymatic activity compared with that of the wild-type enzyme, although this is not always the case (see “Alcohol Dehydrogenase,” below). However, the impact of amino acid substitution(s) on the catalytic activity of a xenobiotic biotransforming enzyme is usually sub- strate-dependent, such that an allelic variant may interact normally with some substrates (and inhibitors) but abnormally with others. The study of the causes, prevalence, and impact of heritable differences in xenobiotic biotransforming enzymes is known as pharmacogenetics.
Biotransformation versus Metabolism
The terms biotransformation and metabolism are often used synonymously, particularly when applied to drugs. For example, xenobiotic biotransforming enzymes are often called drug-metabolizing enzymes. The term xenobiotic biotransforming enzymes is more encompassing, although it conceals the fact that steroids and several other endogenous chemicals are substrates for these enzymes. The term metabolism is often used to describe the total fate of a xenobiotic, which includes absorption, distribution, biotransformation, and elimination. However, metabolism is commonly used to mean biotransformation, which is understandable from the standpoint that the products of xenobiotic biotransformation are known as metabolites. Furthermore, individuals with a genetic enzyme deficiency resulting in impaired xenobiotic biotransformation are described as poor metabolizers rather than poor biotransformers. (Individuals with the normal phenotype are called extensive metabolizers.)
GENERAL PRINCIPLES
Basic Properties of Xenobiotic
Biotransforming Enzymes
Xenobiotic biotransformation is the principal mechanism for maintaining homeostasis during exposure of organisms to small foreign molecules, such as drugs. In general, xenobiotic biotransformation is accomplished by a limited number of enzymes with broad substrate specificities. The synthesis of some of these enzymes is triggered by the xenobiotic (by the process of enzyme induction), but in most cases the enzymes are expressed constitutively (i.e., they are synthesized in the absence of a discernible external stimulus). The specificity of xenobiotic biotransforming enzymes is so broad that they metabolize a large variety of endogenous chemicals, such as ethanol, acetone, steroid hormones, vitamins A and D, bilirubin, bile acids, fatty acids, and eicosanoids.
Indeed, xenobiotic biotransforming enzymes, or enzymes that are closely related, play an important role in the synthesis of many of these same molecules. For example, several steps in the syn-
Stereochemical Aspects of Xenobiotic
Biotransformation
Many xenobiotics, especially drugs, contain one or more chiral centers and can therefore exist in two mirror-image forms called stereoisomers or enantiomers. The biotransformation of some chiral xenobiotics occurs stereoselectively—meaning that one enantiomer (stereoisomer) is biotransformed faster than its antipode. For example, the antiepileptic drug Mesantoin, which is a racemic mixture of R- and S-mephenytoin, is biotransformed stereoselectively in humans, such that the S-enantiomer is rapidly hydroxylated (by a cytochrome enzyme called CYP2C19) and eliminated faster than the R-enantiomer. The ability of some chiral xenobiotics to inhibit xenobiotic biotransforming enzymes can also occur stereoselectively. For example, quinidine is a potent inhibitor of a human cytochrome P450 enzyme called CYP2D6, on which quinine, its antipode, has relatively little inhibitory effect.
In some cases, achiral molecules (or achiral centers) are converted to a mixture of enantiomeric metabolites, and this conver-

CHAPTER 6 BIOTRANSFORMATION OF XENOBIOTICS |
135 |
sion may proceed stereoselectively such that one enantiomer is formed preferentially over its antipode. For example, several cytochrome P450 enzymes catalyze the 6-hydroxylation of steroid hormones. Some P450 enzymes (such as CYP2A1) preferentially catalyze the 6 -hydroxylation reaction, whereas other P450 enzymes (such as CYP3A) preferentially catalyze the 6 -hydroxyla- tion reaction (which is a major route of hepatic steroid biotransformation). Ketones can be reduced by carbonyl reductases to a mixture of enantiomeric secondary alcohols, and this often occurs with a high degree of stereoselectivity. For example, pentoxifylline is reduced by carbonyl reductases in blood and liver to a mixture of secondary alcohols, with the major metabolite having an S-configuration, as shown in Fig. 6-1. Interestingly, the minor metabolite, a secondary alcohol with the R-configuration, has pharmacologic properties distinct from those of its S-antipode and its ketone precursor, pentoxifylline. This minor metabolite is known as lisofylline, which is under clinical investigation for the treatment of various diseases.
The reduction of ketones to secondary alcohols is a reversible reaction, and such interconversions can lead to an inversion of configuration, in which case a secondary alcohol with, say, an R-configuration is oxidized to a ketone (which is achiral); it, in turn, is reduced to a secondary alcohol with an S-configuration (i.e., R-alcohol ketone S-alcohol). This interconversion between achiral ketones and enantiomeric secondary alcohols likely explains why the administration of pure R-albuterol to human volunteers results in the formation of S-albuterol, just as the administration of pure S-albuterol leads to the formation of R-albuterol (Boulton and Fawcett, 1997).
Phase I and Phase II Biotransformation
The reactions catalyzed by xenobiotic biotransforming enzymes are generally divided into two groups, called phase I and phase II, as shown in Table 6-1 (Williams, 1971). Phase I reactions involve hydrolysis, reduction, and oxidation. These reactions expose or introduce a functional group (–OH, –NH2, – SH or –COOH), and usually result in only a small increase in hydrophilicity. Phase II biotransformation reactions include glucuronidation, sulfonation (more commonly called sulfation), acetylation, methylation, conjugation with glutathione (mercapturic acid synthesis), and conjugation with amino acids (such as glycine, taurine, and glutamic acid). The cofactors for these reactions (discussed later) react with functional groups that are either present on the xenobiotic or are introduced/exposed during phase I biotransformation. Most phase II biotransformation reactions result in a large increase in xenobiotic hydrophilicity, hence they greatly promote the excretion of foreign chemicals.
Phase II biotransformation of xenobiotics may or may not be preceded by phase I biotransformation. For example, morphine, heroin, and codeine are all converted to morphine-3-glucuronide. In the case of morphine, this metabolite forms by direct conjugation with glucuronic acid. In the other two cases, however, conjugation with glucuronic acid is preceded by phase I biotransformation: hydrolysis (deacetylation) in the case of heroin and O-demethylation (involving oxidation by cytochrome P450) in the case of codeine. Similarly, acetaminophen can be glucuronidated and sulfated directly, whereas phenacetin must undergo phase I metabolism (involving O-deethylation to acetaminophen) prior to un-
Figure 6-1. Stereochemical aspects of xenobiotic biotransformation: Formation of two enantiomeric metabolites (secondary alcohols) from a single, achiral molecule (a ketone).
Oxidation of a chiral secondary alcohol to an achiral ketone, and subsequent reduction of the ketone to a secondary alcohol of opposite configuration (e.g., R-alcohol ketone S-alcohol) is an example of a process known as inversion of configuration.

136 UNIT 2 DISPOSITION OF TOXICANTS
Table 6-1
General Pathways of Xenobiotic Biotransformation and
Their Major Subcellular Location
|
|
|
REACTION |
ENZYME |
LOCALIZATION |
|
|
|
|
Phase I |
|
|
|
|
Hydrolysis |
Esterase |
Microsomes, cytosol, lysosomes, blood |
|
Peptidase |
Blood, lysosomes |
|
Epoxide hydrolase |
Microsomes, cytosol |
Reduction |
Azoand nitro-reduction |
Microflora, microsomes, cytosol |
|
Carbonyl reduction |
Cytosol, blood, microsomes |
|
Disulfide reduction |
Cytosol |
|
Sulfoxide reduction |
Cytosol |
|
Quinone reduction |
Cytosol, microsomes |
|
Reductive dehalogenation |
Microsomes |
Oxidation |
Alcohol dehydrogenase |
Cytosol |
|
Aldehyde dehydrogenase |
Mitochondria, cytosol |
|
Aldehyde oxidase |
Cytosol |
|
Xanthine oxidase |
Cytosol |
|
Monoamine oxidase |
Mitochondria |
|
Diamine oxidase |
Cytosol |
|
Prostaglandin H synthase |
Microsomes |
|
Flavin-monooxygenases |
Microsomes |
|
Cytochrome P450 |
Microsomes |
|
|
|
|
Phase II |
|
|
|
|
|
Glucuronide conjugation |
Microsomes |
|
Sulfate conjugation |
Cytosol |
|
Glutathione conjugation |
Cytosol, microsomes |
|
Amino acid conjugation |
Mitochondria, microsomes |
|
Acylation |
Mitochondria, cytosol |
|
Methylation |
Cytosol, microsomes, blood |
|
|
|
dergoing phase II biotransformation. These examples illustrate how phase I biotransformation is often required for subsequent phase II biotransformation. In general, phase II biotransformation does not precede phase I biotransformation, although there are exceptions to this rule. For example, some sulfated steroids (including some steroid disulfates) are hydroxylated by cytochrome P450.
Nomenclature of Xenobiotic
Biotransforming Enzymes
The enzymes involved in xenobiotic biotransformation tend to have broad and overlapping substrate specificities, which precludes the possibility of naming the individual enzymes after the reactions they catalyze (which is how most other enzymes are named). Many of the enzymes involved in xenobiotic biotransformation have been cloned and sequenced; in several cases, arbitrary nomenclature systems have been developed based on the primary amino acid sequence of the individual enzymes.
Distribution of Xenobiotic
Biotransforming Enzymes
Xenobiotic biotransforming enzymes are widely distributed throughout the body and are present in several subcellular com-
partments. In vertebrates, the liver is the richest source of enzymes catalyzing biotransformation reactions. These enzymes are also located in the skin, lung, nasal mucosa, eye, and gastrointestinal tract—which can be rationalized on the basis that these are major routes of exposure to xenobiotics—as well as numerous other tissues, including the kidney, adrenal, pancreas, spleen, heart, brain, testis, ovary, placenta, plasma, erythrocytes, platelets, lymphocytes, and aorta (Gram, 1980; Farrell, 1987; Krishna and Klotz, 1994). Intestinal microflora play an important role in the biotransformation of certain xenobiotics. Within the liver (and most other organs), the enzymes catalyzing xenobiotic biotransformation reactions are located primarily in the endoplasmic reticulum (microsomes) or the soluble fraction of the cytoplasm (cytosol), with lesser amounts in mitochondria, nuclei, and lysosomes (see Table 6-1). Their presence in the endoplasmic reticulum can be rationalized on the basis that those xenobiotics requiring biotransformation for urinary or biliary excretion will likely be lipophilic and, hence, soluble in the lipid bilayer of the endoplasmic reticulum.
By extracting and biotransforming xenobiotics absorbed from the gastrointestinal tract, the liver limits the systemic bioavailability of orally ingested xenobiotics, a process known as first-pass elimination. In some cases, xenobiotic biotransformation in the intestine contributes significantly to the first-pass elimination of foreign chemicals. For example, the oxidation of cyclosporine by cytochrome P450 and the conjugation of morphine with glucuronic

CHAPTER 6 BIOTRANSFORMATION OF XENOBIOTICS |
137 |
acid in the small intestine limit the systemic bioavailability of these drugs. Under certain circumstances, oxidation of ethanol to acetaldehyde in the gastric mucosa reduces the systemic bioavailability of alcohol. Some extrahepatic sites contain high levels of xenobiotic biotransforming enzymes, but their small size minimizes their overall contribution to the biotransformation of xenobiotics. For example, certain xenobiotic biotransforming enzymes (such as cytochrome P450 enzymes, flavin monooxygenases, glutathione S-transferases, and carboxylesterases) are present in nasal epithelium at levels that rival those found in the liver. The nasal epithelium plays an important role in the biotransformation of inhaled xenobiotics, including odorants, but is quantitatively unimportant in the biotransformation of orally ingested xenobiotics (Brittebo, 1993).
The fact that tissues differ enormously in their capacity to biotransform xenobiotics has important toxicologic implications in terms of tissue-specific chemical injury. Several xenobiotics, such as acetaminophen and carbon tetrachloride, are hepatotoxic due to their activation to reactive metabolites in the liver (Anders, 1985). Cells within an organ also differ in their capacity to biotransform xenobiotics, and this heterogeneity also has toxicologic implications. For example, the cytochrome P450 enzymes that activate acetaminophen and carbon tetrachloride to their reactive metabolites are localized in the centrilobular region of the liver (zone 3), which is why these xenobiotics cause centrilobular necrosis. Species differences in xenobiotic biotransforming enzymes have both toxicologic and pharmacologic consequences, as do factors that influence the activity of xenobiotic biotransforming enzymes (Kato, 1979). For example, the duration of action of hexobarbital (i.e., narcosis) in mice ( 10 min), rabbits ( 50 min), rats ( 100 min), and dogs ( 300 min) is inversely related to the rate of hexobarbital biotransformation (i.e., 3-hydroxylation) by liver microsomal cytochrome P450, which follows the rank order mouse rabbit rat dog. The duration of action of hexobarbital in rats can be shortened from 100 to 30 min by prior treatment with phenobarbital, which induces cytochrome P450 and thereby increases the rate of hexobarbital 3-hydroxylation. By inducing cytochrome P450, treatment of rats with phenobarbital also increases the hepatotoxic effects of acetaminophen and carbon tetrachloride. Species differences in xenobiotic biotransforming enzymes and factors that affect the pharmacologic or toxicologic effects of xenobiotics are discussed throughout this chapter as each of the major xenobiotic biotransforming enzyme systems is described.
XENOBIOTIC
BIOTRANSFORMATION
BY PHASE I ENZYMES
Phase I reactions involve hydrolysis, reduction, and oxidation of xenobiotics, as shown in Table 6-1. These reactions expose or introduce a functional group (–OH, –NH2, –SH, or –COOH) and usually result in only a small increase in the hydrophilicity of xenobiotics. The functional groups exposed or introduced during phase I biotransformation are often sites of phase II biotransformation.
Hydrolysis
Carboxylesterases, Pseudocholinesterase, and Paraoxonase
Mammals contain a variety of hydrolytic enzymes—namely, carboxylesterases, cholinesterases and organophosphatases—that hy-
drolyze xenobiotics containing such functional groups as a carboxylic acid ester (procaine), amide (procainamide), thioester (spironolactone), phosphoric acid ester (paraoxon), and acid anhydride [diisopropylfluorophosphate (DFP)], as shown in Fig. 6-2 (Satoh and Hosokawa, 1998). The hydrolysis of carboxylic acid esters, amides, and thioesters is largely catalyzed by carboxylesterases (EC 3.1.1.1), which are located in various tissues and serum, and by two esterases in blood: true acetylcholinesterase (EC 3.1.17), which is located on erythrocyte membranes and is a dimeric enzyme (in contrast to the tetrameric form of the same enzyme found in nervous tissue), and pseudocholinesterase (EC 3.1.1.8), which is also known as butyrylcholinesterase and is located in serum. Phosphoric acid esters are hydrolyzed by paraoxonase, a serum enzyme that is known both as an aryldialkylphosphatase (EC 3.1.8.1) or organophosphatase (for its ability to hydrolyze organophosphates such as paraoxon) and as an arylesterase (for its ability to hydrolyze aromatic carboxylic acid esters such as phenylacetate). Phosphoric acid anhydrides, such as DFP, are hydrolyzed by a related organophosphatase, namely diisopropylfluorophosphatase (EC 3.1.8.2). In the presence of an alcohol, carboxylesterases can catalyze the transesterification of xenobiotics, which accounts for the conversion of cocaine (a methyl ester) to ethylcocaine (the corresponding ethyl ester) (Fig. 6-2). Esterases are not the only enzymes capable of cleaving esters. Aldehyde dehydrogenase has esterase activity. Examples are given later in this chapter (under “Cytochrome P450”) in which the cleavage of xenobiotics containing a carboxylic acid ester is catalyzed by cytochrome P450.
Carboxylesterases in serum and tissues and serum cholinesterase collectively determine the duration and site of action of certain drugs. For example, procaine, a carboxylic acid ester, is rapidly hydrolyzed, which is why this drug is used mainly as a local anesthetic. In contrast, procainamide, the amide analog of procaine, is hydrolyzed much more slowly; hence this drug reaches the systematic circulation, where it is useful in the treatment of cardiac arrhythmia. In general, enzymatic hydrolysis of amides occurs more slowly than that of esters, although electronic factors can influence the rate of hydrolysis. The presence of elec- tron-withdrawing substituents weakens an amide bond, making it more susceptible to enzymatic hydrolysis.
In 1953, Aldridge classified esterases based on the nature of their interaction with organophosphates. Esterases that hydrolyze organophosphates are classified as A-esterases; those that are inhibited by organophosphates are classified as B-esterases, whereas those that do not interact with organophosphates are classified as C-esterases. Carboxylesterases and cholinesterase belong to the B- esterase group, which is also known as the family of serine esterase. Organophosphatases such as paraoxonase belong to the A- esterase group.
Although they are not inhibited by organophosphates, A-esterases are inhibited by mercurial compounds that react with free sulfhydryl (–SH) groups, such as para-chloromercuribenzoate (PCMB), whereas the opposite is true of B-esterases (i.e., they are inhibited by organophosphates but not by PCMB). Augustinsson (1966) postulated that the major difference between A- and B- esterases is that the former contain a cysteine residue at their active site, whereas the latter contain a serine residue. This hypothesis has been substantiated in the case of B-esterases but not in the case of A-esterases.
Carboxylesterases and cholinesterases are B-esterases with a serine residue at the active site. The mechanism of catalysis by
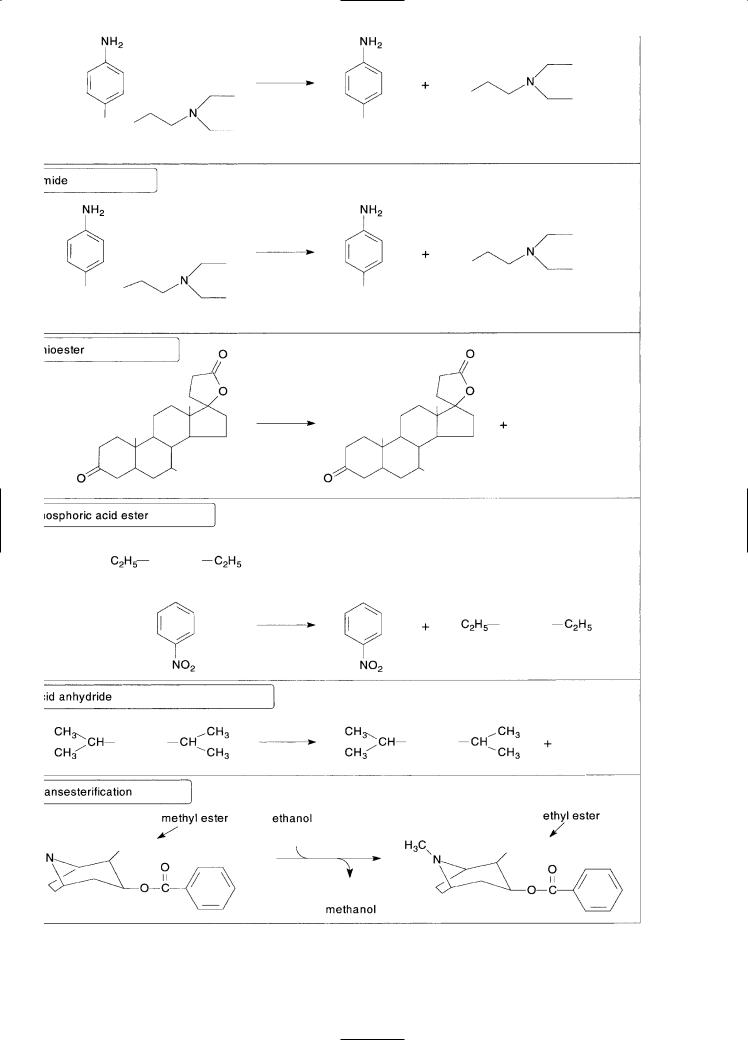
Figure 6-2. Examples of reactions catalyzed by carboxylesterases, cholinesterases and organophosphatases.
138

CHAPTER 6 BIOTRANSFORMATION OF XENOBIOTICS |
139 |
B-esterases is analogous to the mechanism of catalysis by serineproteases. In the case of carboxylesterases, for example, it involves charge relay among a catalytic triad comprised of an acidic amino acid residue [glutamate (Glu335)], a basic residue [histidine (His448)], and a nucleophilic residue [serine (Ser203)] (Yan et al., 1994; Satoh and Hosokawa, 1998). Organophosphates bind to the nucleophilic OH-group on the active site serine residue to form a phosphorus-oxygen bond, which is not readily cleaved by water. Therefore, organophosphates bind stoichiometrically to B-esterases and inhibit their enzymatic activity. The mechanism of catalysis of carboxylesterases is discussed in more detail under “Epoxide Hydrolase,” below.
Based on the observation that A-esterases are inhibited by PCMB but not by organophosphates, Augustinsson (1966) postulated that organophosphates bind to a nucleophilic SH-group on an active site cysteine residue and form a phosphorus-sulfur bond, which is readily cleaved by water. A strong argument against this postulate is the fact that, when the only potential active site cysteine residue in human paraoxonase (Cys283) is substituted with serine or alanine, there is no loss of catalytic activity (Sorenson et al., 1995). Paraoxonase requires Ca2 , both for stability and catalytic activity, which raises the possibility that the hydrolysis of organophosphates by paraoxonase involves metal-catalyzed hydrolysis, analogous to that proposed for calcium-dependent phospholipase A2 or zinc-dependent phosphotriesterase activity (Sorenson et al., 1995).
Esterases play an important role in limiting the toxicity of organophosphates. The mechanism of toxicity of organophosphorus pesticides (and carbamate insecticides) involves inhibition of brain acetylcholinesterase, which is a serine-containing esterase that terminates the action of the neurotransmitter, acetylcholine. The symptoms of organophosphate toxicity resemble those caused by excessive stimulation of cholinergic nerves. The covalent interaction between organophosphates and brain acetylcholinesterase is analogous to their binding to the active site serine residue in all B-esterases. As previously mentioned, organophosphates are hydrolyzed by some esterases (the A-esterases, such as paraoxonase) and bind stoichiometrically and, for the most part, irreversibly to carboxylesterases and cholinesterases. Both types of interactions (i.e., hydrolysis and covalent binding) play an important role in the detoxication of these compounds. Numerous studies have shown an inverse relationship between esterase activity and susceptibility to the toxic effect of organophosphates. Factors that decrease esterase activity potentiate the toxic effects of organophosphates, whereas factors that increase carboxylesterase activity have a protective effect. For example, the susceptibility of animals to the toxicity of parathion, malathion, and diisopropylfluorophosphate is inversely related to the level of serum esterase activity. Differences in the susceptibility of several mammalian species to organophosphate toxicity can be abolished by pretreatment with selective esterase inhibitors such as cresylbenzodioxaphosphorin oxide, the active metabolite of tri-ortho-tolylphosphate (which is also known as tri-ortho-cresylphosphate or TOCP). Esterases are not the only enzymes involved in the detoxication of organophosphorus pesticides. Certain organophosphorus compounds are detoxified by cytochrome P450, flavin monooxygenases, and glutathione S-transferases.
Pseudocholinesterase is a tetrameric glycoprotein (Mr 342 kDa) containing four identical subunits, each having one catalytic site. The enzyme hydrolyzes succinylcholine, mivacurium, procaine, chlorpropaine, tetracaine, cocaine, heroin and other
drugs. The duration of action of the muscle relaxant succinylcholine is determined by serum pseudocholinesterase. In some individuals ( 2 percent of Caucasians), succinylcholine causes prolonged muscular relaxation and apnea, which led to the discovery of an atypical form of pseudocholinesterase (Asp70 Gly70) (La Du, 1992; Lockridge, 1992). Although this atypical enzyme has markedly diminished activity toward succinylcholine (which is the genetic basis for the exaggerated response to this muscle relaxant in affected individuals), it nevertheless has appreciable activity toward other substrates, such as acetylcholine and benzoylcholine. The normal and atypical pseudocholinesterases are equally sensitive to the inhibitory effect of certain organophosphates, but the atypical enzyme is relatively resistant to the inhibitory effect of dibucaine, a local anesthetic, which forms the basis of a diagnostic test for atypical pseudocholinesterase. The discovery of atypical pseudocholinesterase is of historical interest because it ushered in the new field of pharmacogenetics. Since the initial discovery of atypical pseudocholinesterase in late 1950s, several allelic variants of the enzyme have been identified.
Carboxylesterases are 60-kDa glycoproteins that are present in a wide variety of tissues, including serum. Most of the carboxylesterase activity in liver is associated with the endoplasmic reticulum, although considerable carboxylesterase activity is present in lysosomes and cytosol. Carboxylesterases generate pharmacologically active metabolites from several ester or amide prodrugs. For example, lovastatin is converted by liver carboxylesterases to the pharmacologically active metabolite, lovastatin -hydroxyacid, which inhibits HMG-CoA reductase and lowers plasma cholesterol levels. There is some evidence to suggest that therapeutic effect of lovastatin is diminished in individuals with low levels of liver carboxylesterase activity (Tang and Kalow, 1995). Carboxylesterases have also been implicated in the activation of a number of anticancer agents, such as 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyoxycamptothecin (CPT-11), which shows promising activity against a broad range of tumor types, including colorectal and cervical cancer. The anticancer effects of CPT-11 are dependent on its conversion to ethyl camptothecin, which is catalyzed by one or more carboxylesterases. As a result of their ability to hydrolyze prodrugs, carboxylesterases may have clinical applications in the treatment of certain cancers. They might be used, for example, to activate prodrugs in vivo and thereby generate potent anticancer agents in highly selected target sites (e.g., at the surface of tumor cells, or inside the tumor cells themselves). Carboxylesterases might be targeted to tumor sites with hybrid monoclonal antibodies (i.e., bifunctional antibodies that recognize the carboxylesterase and the tumor cell), or the cDNA encoding a carboxylesterase might be targeted to the tumor cells via a viral vector. In the case of CTP-11, this therapeutic strategy would release the anticancer drug, ethyl camptothecin, in the vicinity of the tumor cells, which would reduce the systemic levels and side effects of this otherwise highly toxic drug (Senter et al., 1996).
In addition to xenobiotics, carboxylesterases hydrolyze numerous endogenous compounds, such as palmitoyl-CoA, monoacylglycerol, diacylglycerol, retinyl ester, platelet-activating factor and other esterified lipids. Carboxylesterases can also catalyze the synthesis of fatty acid ethyl esters, which represents a nonoxidative pathway of ethanol metabolism in adipose and certain other tissues. In the case of platelet-activating factor, carboxylesterases catalyze both the deacetylation of PAF and its subsequent esterification with fatty acids to form phosphatidylcholine (Hosokawa and Satoh, 1998).

140 |
UNIT 2 DISPOSITION OF TOXICANTS |
Certain carboxylesterases also have a physiologic function in anchoring other proteins to the endoplasmic reticulum. For example, the lysosomal enzyme -glucuronidase is also present in the endoplasmic reticulum, where it is anchored in the lumen by egasyn, a microsomal carboxylesterase. Egasyn binds to-glucuronidase via its active site serine residue, which effectively abolishes the carboxylesterase activity of egasyn, although there is no corresponding loss of -glucuronidase activity. The retention of-glucuronidase in the lumen of the ER is thought to be physiologically significant. Glucuronidation by microsomal UDPglucuronosyltransferases is a major pathway in the clearance of many endogenous aglycones (such as bilirubin) and xenobiotics (such as drugs). However, hydrolysis of glucuronides by -glu- curonidase complexed with egasyn in the lumen of the endoplasmic reticulum appears to be an important mechanism for recycling endogenous compounds, such as steroid hormones (Dwivedi et al., 1987). The acute-phase response protein, C-response protein, is similarly anchored in the endoplasmic reticulum by egasyn.
A nomenclature system for classifying carboxylesterases has been proposed by Satoh and Hosokawa (1998). The broad substrate specificity of these enzymes precludes the possibility of naming them for the reactions they catalyze, for which reason Satoh and Hosokawa proposed a classification and nomenclature system that is based on a comparison of the amino acid sequences of carboxylesterases (regardless of the species of origin) with the sequence of a human carboxylesterase. Four gene families have been identified (designated CES 1-4) with three subdivisions in the first gene family (namely CES 1A, CES 1B and CES 1C). The first subfamily is further subdivided into three groups (designated CES 1A1, 1A2, and 1A3). Liver microsomes from all mammalian species, including humans, contain at least one carboxylesterase, but the exact number of carboxylesterases expressed in any one tissue or species is not known. Because carboxylesterases are glycoproteins, variations in carbohydrate content can give rise to multiple forms of the same enzyme. Consequently, the large number of carboxylesterases that have been identified by isoelectric focusing and nondenaturing gel electrophoresis probably overestimates the number of genes encoding these enzymes.
Metabolism of xenobiotics by carboxylesterases is not always a detoxication process. Figure 6-3 shows some examples in which carboxylesterases convert xenobiotics to toxic and tumorigenic metabolites.
Peptidases With the advent of recombinant DNA techology, numerous human peptides have been mass-produced for use as drugs, and several recombinant peptide hormones, growth factors, cytokines, soluble receptors, and humanized monoclonal antibodies currently are used therapeutically. To avoid acid-precipitation and proteolytic degradation in the gastrointestinal tract, peptides are administered parenterally. Nevertheless, peptides are hydrolyzed in the blood and tissues by a variety of peptidases, including aminopeptidases and carboxypeptidases, which hydrolyze amino acids at the N- and C-terminus, respectively, and endopeptidases, which cleave peptides at specific internal sites (trypsin, for example, cleaves peptides on the C-terminal side of arginine or lysine residues) (Humphrey and Ringrose, 1986). Peptidases cleave the amide linkage between adjacent amino acids, hence, they function as amidases. As in the case of carboxylesterases, the active site of peptidases contains either a serine or cysteine residue, which initiates a nucleophilic attack on the carbonyl moiety of the amide
Figure 6-3. Activation of xenobiotics to toxic and tumorigenic metabolites by carboxylesterases.

CHAPTER 6 BIOTRANSFORMATION OF XENOBIOTICS |
141 |
bond. As previously noted, the mechanism of catalysis by serine proteases, such as chymotrypsin, is similar to that by serine esterases (B-esterases).
Epoxide Hydrolase Epoxide hydrolase catalyzes the trans- addition of water to alkene epoxides and arene oxides (oxiranes), which can form during the cytochrome P450-dependent oxidation of aliphatic alkenes and aromatic hydrocarbons, respectively. As shown in Fig. 6-4, the products of this hydrolysis are vicinal diols with a trans-configuration (i.e., trans-1,2-dihydrodiols); a notable exception being the conversion of leukotriene A4 (LTA4) to leukotriene B4 (LTB4), in which case the two hydroxyl groups that result from epoxide hydrolysis appear on nonadjacent carbon atoms. Although the levels vary from one tissue to the next, epoxide hydrolase is present in virtually all tissues, including the liver, testis, ovary, lung, kidney, skin, intestine, colon, spleen, thymus, brain, and heart.
There are five distinct forms of epoxide hydrolase in mammals: microsomal epoxide hydrolase (mEH), soluble epoxide hydrolase (sEH), cholesterol epoxide hydrolase, LTA4 hydrolase, and hepoxilin hydrolase (Beetham et al., 1995). As their names imply, the latter three enzymes appear to hydrolyze endogenous epoxides exclusively and have virtually no capacity to detoxify xenobiotic oxides. LTA4 hydrolase is distinct from the other epoxide hydrolases because it is a bifunctional zinc metalloenzyme that has both epoxide hydrolase and peptidase activity and because the two hydroxyl groups introduced during the conversion of LTA4 to LTB4 are eight carbon atoms apart.
In contrast to the high degree of substrate specificity displayed by the cholesterol, LTA4 and hepoxilin epoxide hydrolases, the microsomal and soluble epoxide hydrolases (mEH and sEH) hydrolyze a wide variety of alkene epoxides and arene oxides. Many epoxides and oxides are intermediary metabolites formed during the cytochrome P450-dependent oxidation of unsaturated aliphatic and aromatic xenobiotics. These electrophilic metabolites might otherwise bind to proteins and nucleic acids and cause cellular toxicity and genetic mutations. Generally, these two forms of epoxide hydrolases and cytochrome P450 enzymes have a similar cellular localization. For example, the distribution of epoxide hydrolase parallels that of cytochrome P450 in liver, lung, and testis. In other words, both enzymes are located in the centrilobu-
Figure 6-4. Examples of the hydrolyation of an alkene epoxide (top) and an arene oxide (bottom) by epoxide hydrolase.
Figure 6-5. Stereoselective hydrolyation of stilbene oxide by microsomal and soluble epoxide hydrolase.
lar region of the liver (zone 3), in Clara and type II cells in the lung, and in Leydig cells in the testis. The colocalization of epoxide hydrolase and cytochrome P450 presumably ensures the rapid detoxication of alkene epoxides and arene oxides generated during the oxidation metabolism of xenobiotics.
The microsomal and soluble forms of epoxide hydrolase show no evident sequence identity and, not surprisingly, are immunochemically distinct proteins (Beetham et al., 1995). Although both enzymes hydrolyze a broad spectrum of compounds, they exhibit different substrate specificities. For example, mEH rapidly hydrolyzes epoxides on cyclic systems, whereas sEH has little activity toward these compounds. In rat and mouse, these two enzymes can even be distinguished by their stereoselective hydration of stilbene-oxide; mEH preferentially hydrolyzes the cis-isomer at pH 9.0, whereas sEH preferentially hydrolyzes the trans-isomer at pH 7.4, as shown in Fig. 6-5.
Electrophilic epoxides and arene oxides are constantly produced during the cytochrome P450-dependent oxidation of unsaturated aliphatic and aromatic xenobiotics and are highly reactive to cellular macromolecules such as DNA and protein. Epoxide hydrolases, particularly mEH and sEH, can rapidly convert these potentially toxic metabolites to the corresponding dihydrodiols, which are less reactive and easier to excrete. Thus, epoxide hydrolases are widely considered as a group of detoxication enzymes. In some cases, however, further oxidation of a dihydrodiol can lead to the formation of diol epoxide derivatives that are no longer substrates for epoxide hydrolase because the oxirane ring is protected by bulky substituents that sterically hinder interaction with the enzyme. This point proved to be extremely important in elucidating the mechanism by which polycyclic aromatic hydrocarbons cause tumors in laboratory animals (Conney, 1982). Tumorigenic polycyclic aromatic hydrocarbons, such as benzo[a]pyrene, are converted by cytochrome P450 to a variety of arene oxides that bind covalently to DNA, making them highly mutagenic to bacteria. One of the major arene oxides formed from benzo[a]pyrene, namely the 4,5-oxide, is highly mutagenic to bacteria but weakly mutagenic to mammalian cells. This discrepancy reflects the rapid inactiva-

142 |
UNIT 2 DISPOSITION OF TOXICANTS |
tion of benzo[a]pyrene 4,5-oxide by epoxide hydrolase in mammalian cells. However, one of the arene oxides formed from benzo[a]pyrene, namely benzo[a]pyrene 7,8-dihydrodiol-9,10- oxide, is not a substrate for epoxide hydrolase and is highly mutagenic to mammalian cells and considerably more potent than benzo[a]pyrene as a lung tumorigen in mice.
Benzo[a]pyrene 7,8-dihydrodiol-9,10-oxide is known as a bay-region diolepoxide, and analogous bay-region diolepoxides are now recognized as tumorigenic metabolites of numerous polycyclic aromatic hydrocarbons. A feature common to all bay-region epoxides is their resistance to hydrolyation by epoxide hydrolase, which results from steric hindrance from the nearby dihydrodiol group. As shown in Fig. 6-6, benzo[a]pyrene 7,8-dihydrodiol-9,10-oxide is formed in three steps: Benzo[a]pyrene is converted to the 7,8- oxide, which is converted to the 7,8-dihydrodiol, which is converted to the corresponding 9,10-epoxide. The first and third steps are epoxidation reactions catalyzed by cytochrome P450 or prostaglandin H synthase, but the second step is catalyzed by epoxide hydrolase. Consequently, even though epoxide hydrolase plays a major role in detoxifying several benzo[a]pyrene oxides, such as the 4,5-oxide, it nevertheless plays a role in converting benzo[a]pyrene to its ultimate tumorigenic metabolite, benzo[a]pyrene 7,8-dihydrodiol-9,10-oxide.
Not all epoxides are highly reactive and toxic to the cells that produce them. The major metabolite of carbamazepine is an epox-
ide, which is so stable that carbamazepine-10,11-epoxide is a major circulating metabolite in patients treated with this antiepileptic drug. (Carbamazepine is converted to a second epoxide, which is less stable and more cytotoxic, as shown under “Cytochrome P450,” below.) Vitamin K epoxide is also a nontoxic epoxide, which is formed and consumed during the vitamin K–dependent-carboxylation of prothrombin and other clotting factors in the liver. Vitamin K epoxide is not hydrated by epoxide hydrolase but is reduced by vitamin K epoxide reductase. This enzyme is inhibited by warfarin and related coumarin anticoagulants, which interrupt the synthesis of several clotting factors.
Epoxide hydrolase is one of several inducible enzymes in liver microsomes. Induction of epoxide hydrolase is invariably associated with the induction of cytochrome P450, and several cytochrome P450 inducers, such as phenobarbital and trans-stilbene oxide, increase the levels of microsomal epoxide hydrolase by up to threefold. In mice, the levels of epoxide hydrolase in liver microsomes can be increased by almost an order of magnitude by antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and ethoxyquin. Epoxide hydrolase is one of several preneoplastic antigens that are overexpressed in chemically induced foci and nodules that eventually develop into liver tumors. Several alcohols, ketones, and imidazoles stimulate microsomal epoxide hydrolase activity in vitro. Epoxide hydrolase cannot be inhibited by antibodies raised against the purified enzyme, but it
Figure 6-6. Role of epoxide hydrolase in the inactivation of benzo[a]pyrene 4,5-oxide and in the conversion of benzo[a]pyrene to its tumorigenic bay-region diolepoxide.
Also shown is the role of dihydrodiol dehydrogenase, a member of the aldoketo reductase (AKR) superfamily, in the formation of reactive catechol and ortho-quinone metabolites of benzo[a]pyrene.
