
ECHO 2013 / Transcatheter Aortic and Mitral Valve Replacement The Future is Now 2
.pdf
Hospitalization Through 2 Years
Inoperable Cohort
TAVR |
Standard Tx p value |
|
|
Repeat Hospitalizations (No.) |
78 |
151 |
<.0001 |
Repeat Hospitalizations (%) |
35.0% |
72.5% |
<.0001 |
Days Alive Out of Hospital |
699 [201-720] |
355 [116-712] |
.0003 |
|
Median [IQR] |
||||
|
|
|
||
|
|
|
|
21



TAVR Admission Costs
$78,540
$80,000
$4,978
$60,000 |
|
|
|
|
Mean (median) LOS |
|
|
$30,756 |
|
|
|
||||
|
|
|
|
|
|
||
|
|
|
|
|
(days) |
|
|
|
|
|
|
|
ICU |
4.0 (2.0) |
|
$40,000 |
|
|
Hospital Costs: |
|
Non-ICU |
6.1 (5.0) |
|
|
|
|
|
||||
|
|
|
|
Total |
10.1 (7.0) |
|
|
|
|
|
$73,563 |
|
|
||
|
|
|
|
Post-Procedure |
8.6 (6.0) |
|
|
|
|
|
|
|
|
||
$20,000 |
|
$42,806 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
(N=175) |
|
|
|
|
|
|
|
|
|
|
|
$0
Index Admission Costs
Procedure |
|
Non-Procedure |
|
||
|
||
MD Fees |
|
|

Published Cost Effectiveness
Estimates
Dollars per Life Year or QALY ($thousands)
$300
$250
$200
$150
$100
$50
$0
aspirin MI |
rosuvastatin |
ICD prim |
CRT-D v. |
dabigatran |
PARTNER |
AF ablation |
dialysis |
PCI stable |
LVAD |
prevention |
high-CRP |
prev |
medical Rx |
AF |
Cohort B |
vs. AAD |
|
CAD |
destination |
|
|
|
|
|
|
|
|
|
Rx |

TAVR Categories
(risk is a continuum)
Operable AS patients
Too Sick
Inoperable
High Risk
Low-Intermediate Risk
|
|
|
|
|
|
|
|
90% |
|
|
|
10% FDA Approval |
|||
November 2011

PARTNER Study Design
Symptomatic Severe Aortic Stenosis
ASSESSMENT: High-Risk AVR Candidate
3,105 Total Patients Screened
n = 699 High-Risk
Total = 1,057 patients
2 Parallel Trials:
Individually Powered
Inoperable n = 358
|
|
ASSESSMENT: |
|
|
ASSESSMENT: |
|||
|
Yes |
No |
|
|||||
|
Transfemoral |
|
Transfemoral |
|||||
|
|
|
|
|||||
|
|
|
Access |
|
|
|
Access |
|
Transfemoral (TF) |
Transapical (TA) |
|
|
|
||||
1:1 Randomization |
1:1 Randomization |
1:1 Randomization |
||||||
N = 244 |
|
N = 248 |
N = 104 |
|
N = 103 |
|
|
|
|
|
|
|
|
|
TF TAVR |
|
Standard |
TF TAVR |
|
AVR |
TA TAVR |
|
AVR |
|
Therapy |
|
|
|
n = 179 |
|
|||||
|
|
|
|
|
|
|
n = 179 |
|
|
VS |
|
|
VS |
|
VS |
||
|
|
|
|
|
||||
|
|
|
|
|
|
|||
Primary Endpoint: All-Cause Mortality at 1 yr |
Primary Endpoint: All-Cause Mortality |
|||||||
|
|
(Non-inferiority) |
|
|
Over Length of Trial (Superiority) |
|||
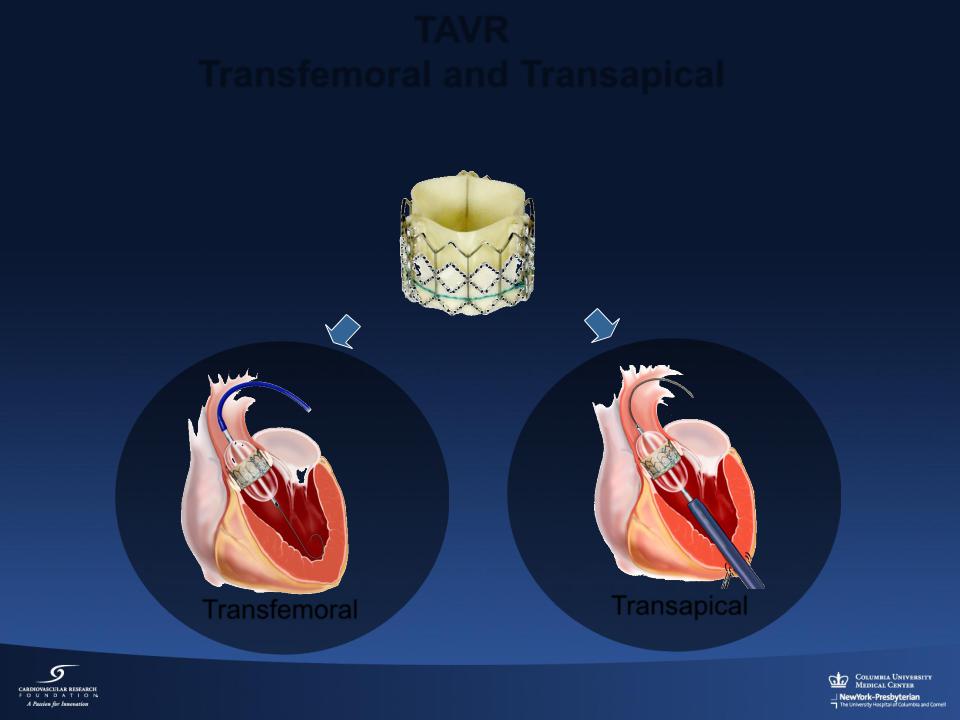
TAVR
Transfemoral and Transapical
Transfemoral |
Transapical |

All-Cause Mortality (ITT)
Landmark Analysis
100%
|
|
|
TAVR |
Mortality starting at 1 yr |
Mortality |
80% |
|
AVR |
HR [95% CI] = |
|
||||
|
|
|||
|
|
|
1.02 [0.74, 1.40] |
|
|
|
|
|
|
|
60% |
|
|
p (log rank) = 0.922 |
|
|
|
|
Cause-All |
40% |
26.8% |
26.3% |
|
|
|
|
|
20% |
24.3% |
24.5% |
|
12.4% |
||
|
|
10.7% |
|
0% |
|
|
|
|
|
|
|
0 |
6 |
12 |
18 |
24 |
30 |
36 |
Numbers at Risk |
|
|
Months |
|
|
|
|
|
|
|
|
|
|
||
TAVR |
348 |
298 |
261 |
239 |
222 |
187 |
149 |
AVR |
351 |
252 |
236 |
223 |
202 |
174 |
142 |

Strokes (ITT)
|
70% |
TAVR |
|
|
|
|
|
|
|
|
HR [95% CI] = |
|
|
|
|
|
60% |
AVR |
|
1.09 [0.62, 1.91] |
|
|
|
|
|
|
p (log rank) = 0.763 |
|
|
||
|
|
|
|
|
|
||
|
50% |
|
|
|
|
|
|
Strokes |
40% |
|
|
|
|
|
|
30% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20% |
|
|
|
7.7% |
|
9.3% |
|
|
|
6.0% |
|
|
||
|
|
|
|
|
|
8.2% |
|
|
10% |
|
3.2% |
|
4.9% |
|
|
|
|
|
|
|
|
||
|
0% |
|
|
|
|
|
|
|
0 |
6 |
12 |
18 |
24 |
30 |
36 |
Months Post Randomization
No. at Risk
TAVR |
348 |
287 |
250 |
228 |
211 |
176 |
139 |
AVR |
351 |
246 |
230 |
217 |
197 |
169 |
139 |
