
ECHO 2013 / Transcatheter Aortic and Mitral Valve Replacement The Future is Now 2
.pdf
MitraClip Implant Experience (2005-2011)
EVEREST II
(Randomized Controlled
Trial)
27%
73%
10%
90%
REALISM Commercial
(Continued Access |
(Europe, Canada, Asia, |
|
Registry) |
Australia) |
|
47% |
|
|
53% |
34% |
66% |
|
|
|
Degenerative |
Functional |
25%
54% 46%
75%
Standard Risk |
*High Risk |
*STS greater than 12 or; Porcelain aorta or mobile ascending aortic atheroma; Post-radiation mediastinum; Previous mediastinitis; Functional MR with EF < 40%; Over 75 years old with EF < 40%; Prior re-operation with patent grafts; Two or more prior chest surgeries; Hepatic cirrhosis; Three or more of the following STS high risk factors: Creatinine > 2.5 mg/dL, Prior chest surgery, Age over 75, EF < 35%

Trial design
~450 patients enrolled at up to 75 US sites
Significant FMR (3+ - 4+ by core lab) Not indicated for mitral valve surgery† Specific anatomical criteria
Randomize 1:1
MitraClip |
Control group |
|
Standard of care |
||
N≈210 |
||
N≈210 |
||
|
Clinical and TTE follow-up:
1, 6, 12, 18, 24, 36, 48, 60 months
†Final definition to be determined in collaboration with FDA
CAUTION: Investigational device. Limited by Federal (U.S.) law to investigational use only.

Transcatheter Mitral Valve Devices
•Leaflets
•MitraClip
•Leaflet ablation
•Space occupier
•Chordal implants
•Transapical
•Transapical-Transseptal
•Annulus
•Coronary sinus annuloplasty
•Direct annuloplasty
•Mechanical cinching
•Energy mediated cinching
•Hybrid
•MV replacement

Mitralign Annuloplasty
Bident (2x2) Procedure
Wire-delivery catheter and wire crossing |
Bident catheter and second wire delivery |
|
Plication and Lock at the P3 site |
Plication and Lock at the P1 and P3 site |

Mitralign Procedure
Pledgets Delivery
Pledget Delivery Catheter crossing the annulus
Courtesy Saibal Kar, MD

Transcatheter MV Implantation:
Challenges
•Delivery
Fold/compress, size (larger than aortics)
•Fixation
More complex structure
No calcium to grab radially
Not a round valve, particularly when diseased and less pliable
Orientation may be important
•Seal
Paravalvular leak likely less well tolerated than aortic (hemolysis)
•Function
LVOT obstruction a concern
Need to preserve the subvalvular apparatus
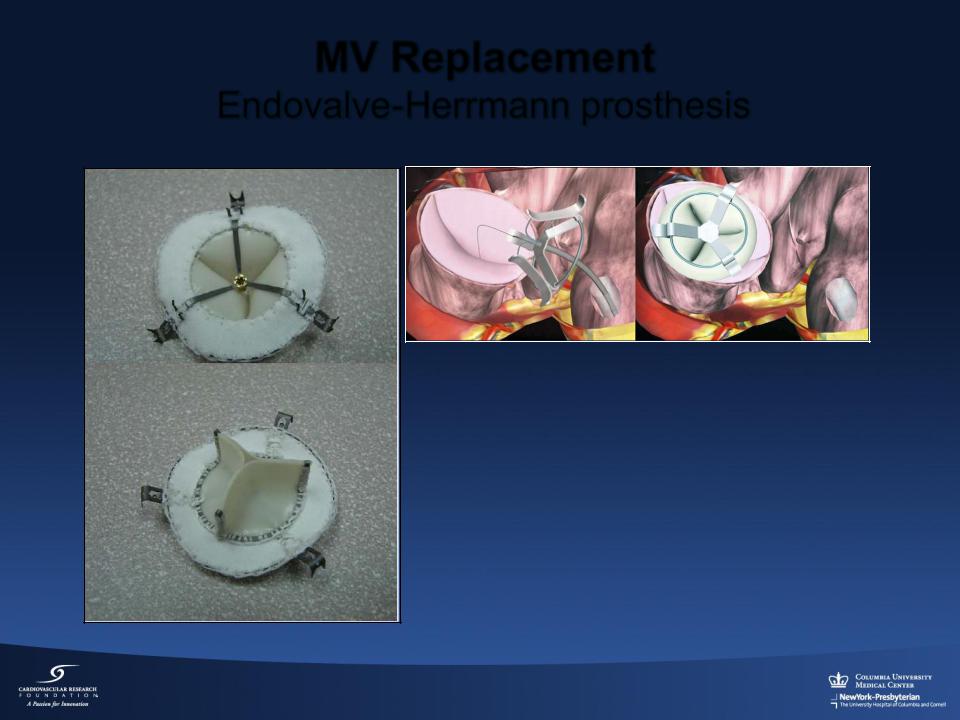
MV Replacement
Endovalve-Herrmann prosthesis
Implant consists of
•Foldable nitinol structure
•Specially designed grippers
•Transapical and Transatrial approaches being evaluated in animal studies
Endovalve Inc. ., Winchester, Massachusetts

MV Replacement
CardiAQ
• Self expanding bi-level |
• Delivered transseptally |
nitinol frame |
• Animal studies |
|
|
• Two sets of opposing anchors |
|
CardiAQ Valve Technologies, Inc., Winchester, Massachusetts

Transapical mitral valved stent implantation
•Electropolished, radially selfexpanding valved stent
•Atrial fixation system connected at 45°
•Tubular portion with bovine pericadial valve (28 mm)
•Ventricular fixation system
•Catheter based delivery system (10.5 mm)
Lutter G, Lozonschi L
J Thorac Cardiovasc Surg. 2010

Final Thoughts
•Proof of principle for both degenerative & functional MR repair with MitraClip Device
Surgical options largely preserved
•EVEREST II randomized trial results in 1 year
Landmark results with 1st percutaneous repair technology to complete a trial
Prospective evaluation of current mitral valve surgery
?role for patients with good surgical option
•Unmet need for poor surgical candidates
High risk randomized trial (COAPT) to begin 2012
•Vs. medical Rx---repeat hospitalizations as primary endpoint
