

4. Photoelectron spectra of amines, nitroso and nitro compounds |
179 |
However, in more complicated amines, this straight correlation is violated. The bicyclic tertiary amine 1-azabicyclo[4.4.4]tetradecane (22) and the acyclic tertiary amine n-Bu3N have nearly the same first IP (7.84 and 7.90 eV, respectively), but the proton affinity of the bicyclic amine is 20 kcal mol 1 lower than that of the acyclic52. On the other hand, for other bridge-head tertiary amines like 1-azabicyclo[2.2.2]octane (quinuclidine, 20) and 1-azabicyclo[3.3.3]undecane (manxine, 21) the expected relation between proton affinities and IP values is observed. The extraordinary properties of 1-azabicyclo[4.4.4]tetradecane (22) are caused by its unusual conformation: the nitrogen lone-pair is directed inward into the bicycle where protonation is not possible. In the protonated form, the strained out-conformation is adopted. This makes it the least basic known tertiary amine with
purely saturated alkyl substituents. Its p |
K |
a, measured in |
ethanol/water, is only |
C0.6 |
93 |
. |
|||
|
|
88 |
. |
|
|||||
Strain effects on amine basicities have been reviewed by Alder |
|
|
|
|
|||||
|
|
|
|
|
|
N |
|
|
|
|
|
|
N |
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
(20) |
(21) |
|
(22) |
|
|
|
|||
Heilbronner and coworkers94 have studied several 2-, 3- and 4-substituted quinuclidines (23 25) by PES and ICR spectroscopy. A linear correlation of the gas-phase basicities and the nN ionization energies relative to the unsubstituted parent molecule was established. Comparison of the solution pKa values with gas-phase basicities revealed that 2-substituted quinuclidines (23) exhibit sizeable solvent-induced proximity effects, i.e. that the corresponding quinuclidinium ions are more acidic in solution than expected on the basis of proton affinities.
|
|
|
R |
|
|
|
R |
|
|
|
R = Me, Cl, CN |
N |
R |
N |
N |
(23) |
|
(24) |
(25) |
E. IP Values and Biological Activity
Houk and coworkers28 have investigated the PE spectra of psychotropic drugs like phenethylamines, tryptamines and LSD and found a correlation between hallucinogenic activity and IP values. Drugs with low IPs are recognized hallucinogens. However, not only first but also second IPs must be taken into account. As examples, human dosage data and average IP values of the first two ionizations (IPav) are given for some drugs in Table 11.
Similarly, the minimal effective brain level, MEBL (nmol/g), required for the drug to interfere with the conditioned avoidance response of rats correlates linearly with IPav. The least-squares correlation is
MEBL D 17.2 ð IPav 132 |
9 |
with a correlation coefficient (r) of 0.95.
180 |
Paul Rademacher |
|
|
|
|
|||
|
TABLE 11. Human dosage data MU and average values |
|
|
|||||
|
of the first two ionizations IPav |
(eV) of some drugsa |
|
|
||||
|
|
|
|
|
IPav |
MUb |
|
|
|
Amphetamine (26) |
|
|
|
9.09 |
0 |
|
|
|
4-Methoxyphenethylamine (27) |
|
|
8.68 |
<1 |
|
|
|
|
3,4-Dimethoxyphenethylamine (28) |
8.44 |
<0.2 |
|
|
|||
|
Mescaline (29) |
|
|
|
8.18 |
1 |
|
|
|
5-Methoxydimethyltryptamine (30) |
7.70 |
>31 |
|
|
|||
|
LSD (31) |
|
|
|
7.64 |
3700 |
|
|
|
|
|
|
|
|
|
|
|
|
aFrom Reference 28. |
|
|
|
|
|
|
|
|
bMescaline units. Activity in humans relative to that of mescaline. |
|
|
|||||
R4 |
|
|
|
R1 |
R2 |
R3 |
R4 |
|
|
|
|
Me |
H |
H |
H |
(26) |
|
|
|
|
|
|||||
R3 |
|
|
|
H |
H |
OMe |
H |
(27) |
|
NH2 |
|
|
H |
OMe |
OMe |
H |
(28) |
2 |
R1 |
|
|
|
|
|
|
|
R |
|
|
|
H |
OMe |
OMe |
OMe |
(29) |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
O |
|
|
|
O |
N |
|
|
|
|
|
|
|
|
Et |
|
|
Me |
|
|||
|
|
|
|
|
||||
Me |
Me |
|
|
N |
|
|
N |
|
|
|
|
|
|
|
|
|
|
Et
N
H
(30)
N
H
(31)
The effect of a biologically active compound is based on its ability to form a complex with a receptor. The intensity of the biological effect is proportional to the stability of this complex, which is dependent on the strength of the interaction of the effector molecule with the active centre of the receptor. The electron structure of the molecule can be decisive for this interaction and this may explain the correlation of ionization potentials and pharmacological properties of certain compounds.
The PE spectra of some other alkaloids like methadone and the opiate narcotics morphine, codeine and heroin have been investigated by Klasinc and coworkers95. Also in this study structure activity relationships based on IPs were sought but not found. Since the interaction of the drug molecule with the receptor is highly specific, it is not unreasonable that the molecular rather than the electronic structure is more important for the physiological activity.

4. Photoelectron spectra of amines, nitroso and nitro compounds |
181 |
F. Transannular Interaction of amino Groups with Other Functional Groups
Strong through-space interactions are possible in medium rings. Such interactions have been discovered in certain alkaloids like cryptopine and protopine, which are characterized by atypical properties of their functional groups like low basicity of amino groups and low carbonyl reactivity of carbonyl groups.
The bicyclic aminoalkene 1-azabicyclo[4.4.4]tetradec-5-ene (32) behaves actually like an enamine96. It is oxidized more readily than its saturated analogues. Protonation does not occur on the nitrogen atom but at the double bond accompanied by transannular cyclization (32 ! 33).
N |
H+ |
N+ |
N |
(32) |
|
(33) |
(34) |
(35)
In order to determine the electronic interaction between the amino and the alkene functionalities, the n and orbital energies of 32 and the two analogous monofunctional compounds 1-azabicyclo[4.4.4]tetradecane (34) and bicyclo[4.4.4]tetradec-1-ene (35) should be known. nN and CDC of 32 and 34 have been determined by PES, while CDC of the unknown alkene 35 has been estimated. The data are depicted in Figure 9.
In the difunctional aminoalkene 32 the CDC MO is destabilized relative to that of alkene 35 by ca 0.5 eV while the nN orbital is stabilized relative to that of amine 35 by the same amount. This is the largest nN/ CDC interaction known so far.
In the monocyclic aminoalkenes 36 39 with an exocyclic CC double bond, only for the eight-membered ring compounds 37 has sizeable nN/ CDC interaction been detected by PES48,97. The through-space interaction of these orbitals in aminoalkenes was found to increase exponentially as their distance decreases97.
CH2 |
CH2 |
|
|
|
|
CH2 |
CH2 |
||
|
|
|||
|
|
|
||
|
N |
N |
N |
|
N |
|
|||
R |
|
|||
CH3 |
CH3 |
|||
|
||||
CH3 |
|
|||
|
|
|||
R = Me, Et, i-Pr, t-Bu |
|
|
||
|
|
|
||
(36) |
(37) |
(38) |
(39) |

182 |
Paul Rademacher |
εi (eV)
−7.30
−7.85
nN
−8.10
πC C
−8.60
Ν |
Ν |
FIGURE 9. Orbital correlation diagram for 1-azabicyclo[4.4.4]tetradec-5-ene (32), 1-azabicyclo[4.4.4]- tetradecane (34) and bicyclo[4.4.4]tetradec-1-ene (35)
Also, the transannular interactions between amino and carbonyl groups in aminoketones, like 40 44, were studied by PES48. Pronounced stabilization of the nN orbital and destabilization of the nO orbital was established by comparison of the relevant ionization potentials with those of the corresponding monofunctional compounds. The shift of the nO orbital was noticed as the best indicator of transannular nN/ CDO interaction and the maximum value was again found for the system with an eight-membered ring (41).
These investigations, which can be considered as relevant for the modelling of analogous bimolecular reactions, have been reviewed recently97.
Martin and coworkers47 have recorded and analysed the PE spectra of 7-azanorbornane (45), 7-azanorbornene (46) and 7-azanorbornadiene (47) as well as of related urethanes. Of prominent interest are the orientation of the lone-pair in 46 (syn or anti), the throughspace interaction with bonds in 46 and 47, and the participation of bond orbitals. For 45 an IP (nN) value of 9.00 eV has been measured. The nN orbital in this molecule has high contributions of the C N bonds and of the anti-oriented C1 C2 and C3 C4bonds. These orbitals mix into the lone-pair of nitrogen in an antibonding way, which causes the comparatively low ionization energy. The syn form is the prevailing conformer of 46. There is direct interaction (homoconjugation) between nN and CDC leading to the splitting of the two highest occupied levels (8.75 and 9.73 eV), which is enforced by interactions with bond orbitals. In 47 (8.60, 9.40 and 10.50 eV) there is strong

4. Photoelectron spectra of amines, nitroso and nitro compounds |
183 |
|||
O |
O |
O |
O |
|
|
N |
N |
N |
|
N |
R |
CH3 |
CH3 |
|
|
|
|
|
|
CH3 |
R = Me, Et, i-Pr, t-Bu, |
|
|
|
|
|
|
|
|
|
4-CH3 -C6 H4 |
|
|
|
(40) |
(41) |
(42) |
(43) |
|
|
|
|
O |
|
N
CH3
(44)
interaction between the lone-pair and two bonds. The n character increases from the highest to the third highest level. This is consistent with the relative band intensities in the HeI and HeII PE spectra of 47.
H H H H
N N N N
(45) |
(46-syn) |
(46-anti) |
(47) |
H
N
G. Diamines
In diamines there are two ionizations associated with the electron lone-pairs. Even when the nitrogen atoms have equal substituents or are related by symmetry, the two IPs are different in energy. The two eigenvalues are not degenerate because the point group of a diamine does not allow this.

184 |
Paul Rademacher |
Linear combination of the two lone-pair orbitals (n1 and n2) with reference to the
relevant symmetry operation leads to a symmetric (nC ) and an antisymmetric combination (n ).
nC D 1/ |
p |
|
n1 C n2 |
(10) |
|
2 |
|||
n D 1/ |
p |
|
n1 n2 |
(11) |
|
2 |
The energy difference n of nC and n is attributed to the interaction of n1 and n2. n is dependent on the separation of the two nitrogen atoms by the number of bonds and also on their mutual geometrical orientation.
PE spectra of diamines have been reviewed briefly by Alder and Sessions98.
The nitrogen lone-pairs can interact both directly through-space and by mixing with other or bonds in the molecule (through-bond interactions)99 which may lead to n values of more than 2 eV. For bicyclic diamines with both nitrogen atoms in bridgehead positions, the relative contributions of the two modes of interaction as a function of
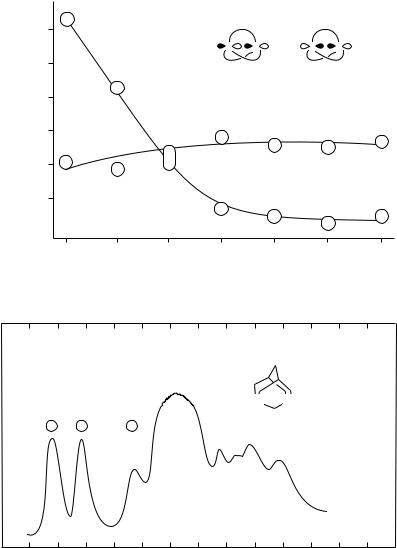
bridge-size have been studied52,100. 1,4-Diazabicyclo[2.2.2]octane (DABCO, 48) forms an unusually persistent radical cation in solution (t1/2 ³ 1 a in CH3CN at 20 °C)101 and shows two bands at 7.52 and 9.65 eV in its PE spectrum102,103. This large split of the two nN ionizations is caused by effective through-bond interaction of the two lone-pairs via the three C C bonds, placing nNC above nN . Homologous diamines (49 54) show much smaller energy differences in their first two IPs, and in most cases the first IP relates to nN (see Figure 10).
N |
N |
|
N |
N N |
N |
N |
N |
|
(48) |
|
(49) |
(50) |
|
(51) |
|
|
N |
N |
N |
N |
N |
|
N |
|
(52) |
|
|
(53) |
|
(54) |
|
The first IPs of these diamines are exceptionally low. In solution too these compounds are easily oxidized giving long-lived radical cations, which are presumed to contain 3- electron bonds. This is in accordance with IP nNC > IP nN , i.e. through-space interaction dominates any through-bond effects.
Lone-pair (n/n) interactions have been studied exhaustively on various diamines98,104 106 including 1,3-diamines and imidazolidines. Since n/n interactions usually are conformation-dependent66,67, from the PE spectra some information about the conformational properties of such compounds may be obtained. A thorough data compilation has been published by Mayer and Martin107.
As another example, in Figure 11 the PE spectrum of 1,3-diazaadamantane (55)108 is depicted. There are two well separated nN ionization bands at 7.75 (nN ) and 8.78 eV
4. Photoelectron spectra of amines, nitroso and nitro compounds |
185 |
||||||
9.5 |
|
|
|
|
|
|
|
|
|
|
N |
N |
N |
N |
|
9.0 |
|
|
|
n − |
|
n + |
|
8.5 |
|
|
|
|
|
|
|
I mj (eV) |
|
|
|
|
|
|
|
8.0 |
|
|
|
|
(n+)−1 |
|
|
7.5 |
|
|
|
|
|
|
|
7.0 |
|
|
|
|
(n−)−1 |
|
|
|
|
|
|
|
|
|
|
[2.2.2] |
[3.2.2] |
[3.3.2] |
[3.3.3] |
[4.3.3] |
[4.4.3] |
[4.4.4] |
|
FIGURE 10. Vertical ionization energies for the first two bands in the PE spectra of bicyclic bridgehead diamines: [2.2.2] = 1,4-diazabicyclo[2.2.2]octane (48), etc. Reprinted with permission from Reference 52. Copyright (1981) American Chemical Society
Countrate
N N
1 |
2 |
3 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
IP (eV)
FIGURE 11. PE spectrum of 1,3-diazaadamantane (55). Reprinted from Reference 108 with kind permission from Elsevier Science Ltd
(nNC ). The splitting is dominated by through-space interaction. In 6-methylene-1,3- diazaadamantane (56) and 1,3-diazaadamantan-6-one (57) Gleiter and coworkers108 have also investigated the participation of another functional group in the n/n interaction. In 56 the two nN orbitals are a little destabilized (ca 0.1 eV) relative to 55, whereas in 57 the inductive effect of the CO group leads to a stabilization of ca 0.5 eV. The ionization

186 |
Paul Rademacher |
potentials of azaadamantanes have recently been calculated by Galasso109 in a Green’s function ab initio study.
N |
N |
N |
N |
N |
N |
(55) |
(56) |
O |
(57) |
The PE spectra of four 2-substituted 1,3-dimethylimidazolidines (58) have been recorded and analysed using AM1 and PM3 quantum chemical calculations106. A single broad band is found for the two nN ionizations and the energies of the two nN orbitals are split by only 0.0 0.3 eV indicating little n/n interaction. This is consistent with envelope conformations of the five-membered ring of 58 and an axial-equatorial orientation of the two N-methyl groups.
CH3 |
|
|
|
|
|
N |
R1 |
|
|
|
|
|
R2 |
|
|
|
|
N |
R1 |
Me |
t-Bu |
Ph |
PhMe C |
|
R2 |
|
|
|
2 |
CH3 |
Me |
H |
H |
H |
|
|
|
|
|
|
|
(58) |
|
|
|
|
|
H. Polyamines
The PE spectra of some cyclic triamines with the nitrogen atoms in 1,3-positions like hexahydro-1,3,5-triazines (59) and tetracyclic derivatives such as 60 and 61 have been studied and interpreted with regard to their conformational properties110. The nN ionizations lead to two strongly overlapping bands between 7.5 and 9.5 eV which are consistent with diequatorial-monoaxial nitrogen substituent orientations at the hexahydro- 1,3,5-triazine rings.
|
|
|
N |
N |
N |
N |
|
N |
|
R |
|
|
|
|
|
N |
|
|
N |
R N R |
|
N |
|
|
|
|
|
||
R = Me, Et, i-Pr, t-Bu |
|
|
|
|
(59) |
|
(60) |
(61) |
|
The azaadamantanes with nitrogen atoms in bridgehead positions present an interesting series of compounds with 1,3-n/n interactions in a rigid cage. The PE spectrum of

4. Photoelectron spectra of amines, nitroso and nitro compounds |
187 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FIGURE 12. PE spectrum of urotropine (62), with assignments. Reprinted from Reference 111 with kind permission from Elsevier Science Ltd
urotropine (1,3,5,7-tetraazaadamantane, 62) is depicted in Figure 12. The unique feature of this molecule is, of course, the presence of four tetrahedrally arranged nitrogen lonepairs (point group Td). These occupy the orbitals 7t2 and 5a1 of which the former is threefold degenerate and is occupied by six electrons. The first ionization band (8.4 eV) is readily identified as the 7t2 lone-pair combination. The band exhibits no significant indication of a Jahn-Teller distortion. There is then a large gap between this band and the broad unresolved composite band commencing at 11.8 eV with a maximum at ca 12.7 eV, indicating that the 7t2/5a1 lone-pair splitting amounts to ca 4.3 eV. This suggests that the through-space interaction is enhanced by coupling with the C N bonds109,111.
Me
N N
N  N N
N N  N
N
N N
(62)(63) (64)
1,3-Diazaadamantane (55) was mentioned in the preceding chapter. In 1,3,5- triazaadamantane the three lone-pairs occupy a doubly degenerate orbital of e symmetry and an a1 MO. Accordingly, two bands for the n ionizations are expected in the PE spectrum, which has not yet been measured. However, the experimental data for its 7- methyl derivative (63) are 8.08 and 9.90 eV104, which can be expected to be quite close to those of the parent molecule. The average IP(nN) values of the azaadamantanes exhibit the variation 7.94 eV (1-azaadamantane54, 64, Table 7), 8.27 eV (55), 8.69 eV (63) and 9.49 eV (62). This sequence reflects the increasing stabilization of the lone-pair orbitals as a consequence of more effective interaction within the cage. The electrochemical oxidation potentials112 of these compounds parallel their first IPs: 0.86 V and 7.75 eV (55), 1.02 V and 8.08 eV (63), 1.37 V and 8.4 eV (62).

188 |
Paul Rademacher |
I. Miscellaneous Amino Compounds
Compounds in which the amino group is directly connected with another functional group like hydrazines, hydroxylamines, enamines and amides were already briefly mentioned in Section II.C. In several cases it has been shown that orbital interactions of these systems can be used for conformational analysis by PES66,67.
Alkaloids have been mentioned in Section II.E.
PES has been applied to study biologically active molecules with amino groups and their constituents like nucleic bases and related compounds (e.g. adenine, guanine, thymine, cytosine, hypoxanthine and their methyl derivatives)113 120 and amino acids92,121,122 or their methyl esters123.
III. NITROSO COMPOUNDS
Solutions of many organic nitroso compounds, R NO, display blue or blue-green colours owing to a weak absorption in the visible region ( ³ 700 nm, ε < 50). In the crystalline state, however, most of these compounds are colourless or at most pale yellow. The reason for this phenomenon is the dimerization reaction124. The dimers have an NN distance of about 131 pm, which indicates a certain degree of double-bond character, and show E/Z isomerism. They may thus be termed diazene-1,2-dioxides.
|
|
R |
O |
R |
R |
2 RNO |
|
|
N N |
or |
N N |
|
|||||
|
|
O |
R |
O |
O |
PE spectroscopic studies of C-nitroso compounds have sometimes been hampered by these properties, but also the dimer monomer transformation has been studied by this technique125,126 (vide infra).
A. Nitrosomethane and Other Nitrosoalkanes
The HeI PE spectrum of nitrosomethane (Figure 13) was first studied by Bergmann and Bock125,127. This compound as well as several other aliphatic and aromatic C-nitroso compounds were investigated by Pfab and coworkers126,128, however several of them were dimers.
Nitrosomethane, CH3NO, has twelve occupied molecular orbitals, of which ten are of a0 and two of a00 symmetry. Of these the outer six valence MOs appear in the HeI range. Some of these orbitals are depicted in Figure 14. The energetic order of the three highest occupied MOs was predicted as nC 9a0 < 2a00 < n 10a0 125 127. The -type orbital is the NDO, and nC and n are the in-phase and the out-of-phase combination, respectively, of oxygen and nitrogen lone-pairs. These orbitals can be considered as the characteristic MOs of C-nitroso compounds and the corresponding IPs should be identified in the PE spectra. In Table 12 the observed ionization potentials are summarized together with the relevant results of quantum chemical calculations.
The PE spectrum of nitrosomethane shows three distinct ionization regions: a separate band at low energy (IPa D 9.15, IPv D 9.76 eV126) followed by two intense and broad composite bands with maxima at 13.9 and 16.3 eV. The assignment of the first band as arising from n (10a0) is unambiguous, whereas to the second band (2a00 ) and nC (9a0) contribute, and the third band originates from (1a00 ) and perhaps a ionization. The two MOs of MeNO are the in-phase and the out-of-phase combinations of NDO and a pseudo orbital of the methyl group ( Me).
