

4. Photoelectron spectra of amines, nitroso and nitro compounds |
169 |
RX is a parameter characterizing the homologous series RX. The values of R are direct measures of the polar inductive effects of alkyl groups relative to that of methyl and correlate well with Taft’s Ł values. Substituent-induced IP shifts can thus be handled by linear free energy relationships (LFER) of the Hammett -type.
Nelsen41 extended such correlations to cyclic compounds for which R is not defined, by introducing a parameter neff representing the ‘effective number of carbon atoms’ in the alkyl groups (equation 5).
R
neff D 1 C 5
Et
With these parameters the first IP of tertiary amines can be calculated (equation 6).
IPcalc D 8.92 0.13 neff |
6 |
The deviation of the experimental IP values from that predicted by equation 6 for ordinary amines is only š0.03 eV, which is about the size of the experimental error in measuring IP. Larger deviations indicate significant changes of the bond angles at the nitrogen atom, and equation 7 has been proposed to estimate the average C N C bond angle ˛ of a tertiary amine from its ionization potential41.
˛ D 110.8 10.5 |
7 |
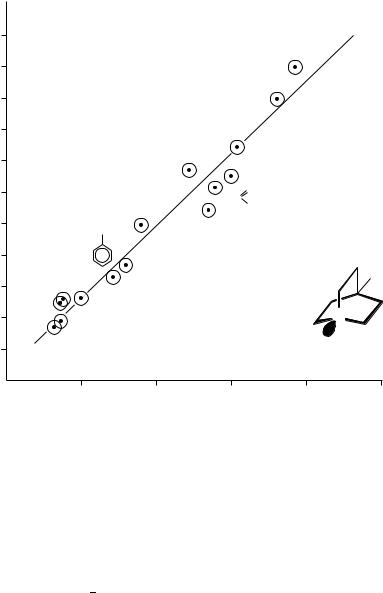
Obviously the nN ionization energy is a function of the type, number and position of the substituents. The IP(nN) values of 4-substituted quinuclidines correlate linearly with Taft’s Ł values51 (Figure 3).
In piperidine the electron lone-pair can occupy either an axial or an equatorial position; in 1-methylpiperidine the axial orientation (1b) is favoured by 99:1 over the equatorial (1a). PE spectra and ab initio calculations on methylpiperidines indicate that axial 2- methyl substituents lower the amine lone-pair ionization potential by about 0.26 eV, while equatorial 2-methyl substituents as well as methyl groups on carbon atoms 3 and 4 lower the lone-pair IP by less than 0.1 eV63. This establishes the mechanism of stabilization of the amine radical cation as hyperconjugative electron release, which is larger for CC bonds than for CH bonds. The anti-periplanar orientation of the nitrogen lone-pair and the vicinal C Me bond (1c) is much more favourable for this type of interaction than the synclinal orientation (1d).
Me |
|
|
|
|
|
|
Me |
N |
N Me |
N |
N |
|
|
|
Me |
|
|
Me |
|
|
|
Me |
|
(1a) |
(1b) |
(1c) |
(1d) |
The nN ionizations of tertiary aliphatic amines (Table 5) are mostly lower than 9 eV and thus small enough for an electron donor in charge-transfer (CT) interactions. Mutai and collaborators64 have studied intramolecular CT interaction in a series of 1-(ω- dimethylaminoalkyl)-4-nitrobenzenes.
O2N |
(CH2)n NMe2 |
|
n = 1-3 |
170 |
Paul Rademacher |
Iv(n)
8.9
8.8
8.7
8.6
8.5
8.4
8.3
8.2
8.1
8.0
7.9
7.8
|
|
|
|
−NO2 |
|
|
|
|
−C ≡ N |
|
|
−OH |
|
−Cl |
|
|
|
|
|
|
|
|
|
−Br |
|
|
|
−C |
O |
|
|
|
OCH3 |
|
|
|
|
|
|
|
|
|
−I |
|
|
|
−C ≡CH |
|
|
|
|
−CH2OH |
|
R |
|
−Me |
|
|
|
|
|
|
|
|
−Et |
−H |
|
|
|
|
|
|
N |
|
|
−i Pr |
|
|
|
|
|
|
|
|
−t Bu |
|
|
|
|
|
0 |
0.5 |
1.0 |
1.5 |
|
|
σ |
|
|
FIGURE 3. Regression of lone-pair ionization energies of 4-substituted quinuclidines vs Taft’s Ł values of the substituents. Reproduced with permission from Reference 12
However, the first ionization bands in the PE spectra of these compounds (vertical IPs are n D 1: 8.67 eV, n D 2: 8.61 eV, n D 3: 8.50 eV) show no apparent anomaly which might be ascribed to intramolecular n/ type CT interaction. This fact suggests that the CT interaction found by UV spectroscopy is weak and that the molecules are present in their open-chain forms under the experimental conditions employed for recording the PE spectra.
Morishima and coworkers30 have studied the CT complexes of iodine with various cyclic and bicyclic amines containing non-adjacent double bonds or aromatic rings. They could show that the iodine amine CT absorption is a useful means of assigning the nN ionizations in the PE spectra of amines.
For some amines it can be very difficult if not impossible to measure the IP(nN) value because of overlap by other strong ionization bands. Benzylamine, PhCH2NH2, is an example. Its PE spectrum26,65 exhibits two strong overlapping bands in the lower energy region (<12 eV) with maxima at 9.03 and 9.39 eV. The corresponding ionizations are most probably related to the aromatic MOs ( 2 and 3). The second band has a hardly noticeable shoulder at about 9.7 eV which might originate from the nN ionization.

4. Photoelectron spectra of amines, nitroso and nitro compounds |
171 |
C. Aromatic Amines
Large changes of the electronic structure and, in particular, of the nN orbital occur when the amino group is directly attached to another functional group with n or orbitals. Such systems have typical electronic, structural and chemical properties and will not be dealt with here in detail. Examples are hydrazines, hydroxylamines, enamines and amides. The most interesting feature of these systems which has been studied by PES are conformationdependent orbital interactions66,67. A special case are the aromatic amines which are not considered as a unique class of compounds although, owing to n/ conjugation, their properties deviate considerably from those of aliphatic amines.
1. Anilines
The simplest aromatic amine, aniline, is a much weaker base than aliphatic primary amines. Also, its structure deviates from that of the latter amines: The sum of the bond angles at the nitrogen atom of aniline is 345°, while in primary aliphatic amines it is about 330°, and the NH2 group is tilted by 37.5 š 2° against the plane of the ring68,69. This partial planarization of the nitrogen atom is caused by delocalization of the nitrogen lone-pair by nN/ Ph orbital interaction. As a sole symmetry element the molecule has a symmetry plane bisecting the amino group and the benzene ring and it belongs to the point group Cs. Since there is only minor deviation from C2v symmetry (only the atoms of the amino group do not lie in the ring plane69) sometimes this point group is used to approximately describe properties of aniline.
Aniline has 50 electrons, thus there are 25 doubly occupied MOs. Seven of these belong to the 1s electrons of the C and N atoms and the remaining 18 are valence-shell MOs. Some of these are depicted in Figure 4. Aniline is one of the most important basic organic compounds, and its PE spectrum (Figure 5) has been investigated by several authors, e.g., (see, References 21,42,70 76). A HeII spectrum was published by Palmer and coworkers75. The relevant data are summarized in Table 9. Because of the interaction of the nitrogen lone-pair with the electrons of the benzene ring the MOs 2 and 3, which are degenerate in benzene, are split. The first and the third ionization band of aniline correspond to the out-of-phase and the in-phase combination of nN and 3, while
π
π |
π |
FIGURE 4. MOs of aniline (AM1 results)

172 |
Paul Rademacher |
FIGURE 5. PE spectrum of aniline
TABLE 9. Ionization potentials IP (eV) and orbital energies ε (eV) of aniline
IP26 |
εa |
εb |
εc |
Ionic stated |
MO |
8.07 |
7.95 |
8.52 |
8.14 |
17a0 (3b1) |
3 3-nN |
9.20 |
9.10 |
9.65 |
9.32 |
8a00 (1a2 ) |
2 |
10.80 |
11.91 |
11.54 |
10.71 |
16a0 (2b1) |
nN 3 C nN |
11.74 |
13.23 |
11.77 |
11.07 |
7a00 (8b2 ) |
|
12.39 |
13.54 |
12.31 |
11.56 |
15a0 (13a1 ) |
|
|
14.35 |
13.81 |
12.83 |
14a0 (1b1) |
1 |
14.05 |
15.82 |
14.10 |
13.27 |
13a0 (7b2) |
|
|
16.15 |
14.70 |
13.82 |
12a0 (12a1 ) |
|
|
16.21 |
14.60 |
13.71 |
6a00 (6b2 ) |
|
15.52 |
17.53 |
16.64 |
15.58 |
11a0 (11a1 ) |
|
15.9 |
18.57 |
17.29 |
|
5a00 (5b2 ) |
|
16.75 |
19.14 |
17.89 |
|
10a0 (10a1 ) |
|
19.0e |
|
22.53 |
|
9a0 (9a1) |
|
22.5e |
|
23.26 |
|
4a00 (4b2 ) |
|
a Ab initio [4-31G]21. bAM126.
c OVGF(AM1)26.
d CsC2v symmetry. eFrom Reference 75.

4. Photoelectron spectra of amines, nitroso and nitro compounds |
173 |
the second band corresponds to 2. For simplicity reasons, the HOMO and HOMO- 2 are termed 3 and nN, respectively. Somewhat more difficult is the identification of the ionization related to 1 since it is superposed by a strong ionization. Relative to benzene21, 1 and 2 are unshifted, while 3 is destabilized by 1.2 eV, which reflects the electron-donating effect of the amino group. Similar relations are also found for the unoccupied MOs of aniline which were studied by Jordan’s group77 and by Distefano and coworkers78 by electron transmission spectroscopy (ETS). The results are reproduced graphically in Figure 6.
In Table 9, the IP values of aniline are compared with orbital energies obtained by quantum chemical calculations. The agreement is sufficient for the assignments even for most of the higher IPs, although the sequence of 6a00 and 12a0 is inverted by the semi-
empirical methods relative to the ab initio results. The smallest deviations |
|
at least for |
||||||||||||||
|
||||||||||||||||
the lower IPs |
|
is found for the outer valence Green’s function (OVGF)19 |
technique, |
|||||||||||||
|
||||||||||||||||
coupled with semi-empirical AMl18 calculations. |
a value of 7.7206 |
|
0.0002 eV has |
|||||||||||||
By zero kinetic energy (ZEKE) PE spectroscopy |
š |
|||||||||||||||
79 |
|
|
|
|
|
|
|
|||||||||
been determined for the first adiabatic IP of aniline |
|
. This technique makes it possible |
||||||||||||||
to obtain accurate and detailed information about molecular ions. |
|
|
|
|
|
|||||||||||
6 |
|
4.82 |
|
|
4.95 |
5b1 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
b2g |
|
|
|
|
|
|
|
|
|
|||
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
εi (eV) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
1.79 |
4b1 |
|
|
|
|
|
||||
|
1.12 |
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
e2u |
|
|
|
|
|
2a2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1.21 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
0 |
|
|
|
|
|
|
−8.07 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
−8 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
3b1 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
e1g |
−9.25 |
|
|
|
1a2 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
−10 |
|
|
|
|
|
|
−9.20 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
−12 |
|
−12.38 |
|
−12.39 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
a2u |
|
|
|
|
|
1b1 |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
FIGURE 6. Correlation diagram for the occupied and vacant MOs of benzene and aniline. Values from References 21 and 78

174 |
Paul Rademacher |
In Table 10 the first three IPs of some substituted anilines are summarized. Aniline and other aromatic amines are systems well suited for studies of substituent effects on the electronic structure and the effects of steric inhibition to resonance42,71,72,74,75,80,81 . Since such effects mainly affect the electronic structure of the benzene ring it would lead too far covering these compounds here in detail. However, a few remarks seem to be adequate. Because of the electron delocalization there are several MOs with substantial nN contributions. However, in most cases the third highest occupied MO (HOMO-2), or the third IP, is mainly related to the electron lone-pair. Therefore, the assignment of the IPs is generally the same as for the parent molecules: IP1 and IP2 are assigned to 3 and2, respectively, and IP3 is assigned to nN.
In the toluidines (methylanilines) the nN MO is destabilized by 0.2 0.3 eV, while in the corresponding monofluoroanilines it is stabilized by about 0.1 eV75. The effect of several fluorine atoms seems to be additive, as indicated by the IP(nN) value of pentafluoroaniline42,71 which is 0.7 eV greater than that of aniline.
If only N-substituted aniline derivatives are considered, the IP( 2) value is observed close to that of the parent compound and to vary in a rather narrow range (8.8 9.1 eV). On the other hand, for IP( 3) (7.0 7.7 eV) and IP(nN) (9.5 10.3 eV) larger deviations relative to unsubstituted aniline and greater variation with substitution are found. This can be explained by perturbations of the corresponding benzene MOs by the substituents which is minimal for 2, because it has no coefficients on C-1 and N (Cs symmetry). In asymmetric aniline derivatives also 2 is expected to interact to a substantial extent
with nN.
Steric resonance inhibition has been ascertained for N-methyl- and N,N- dimethylanilines with further substituents in the ortho positions42,72. By considering the changes in the first three orbital levels as observed by PE spectroscopy, the amount by which the nitrogen lone-pair electrons are twisted about the N-phenyl bond can be estimated. For example, the dihedral angle of N,N-dimethyl-2,6-dimethylaniline (2) was estimated at 30 46° from the lone-pair ionization energy as well as from the split of 2 and3. These ‘classical’ investigations by Maier and Turner42 in the field of conformational analysis by PE spectroscopy have been reviewed previously66,67.
N φ |
N |
N |
N |
(2) |
(3) |
(4) |
(5) |
Julolidine (3) and benzoquinuclidine (4) can be considered as aniline derivatives with parallel and perpendicular electron lone-pairs, respectively. Relative to N,N- dimethylaniline (5), the simplest tertiary aromatic amine, the nN orbital of julolidine is destabilized by 0.20 eV, while that of benzoquinuclidine is destabilized by 0.80 eV42. In the latter compound there is no n/ conjugation while in the former it has a maximum value and, accordingly, the splitting of the first and the third IP is much smaller (0.70 eV) than in the former (2.55 eV) compound.
Rettig and Gleiter81 have studied the dependence of intramolecular rotation in 4-cyano- N,N-dialkylanilines 6 12 on the twist angle by fluorescence, UV absorption and PE spectroscopic measurements. The twist angles were determined from the split of the first and the third IP. While in molecules 6, 8 and 9, 11 and 12 the twist of the amino group

|
4. Photoelectron spectra of amines, nitroso and nitro compounds |
175 |
||||||||||
TABLE 10. Ionization potentials (eV) of substituted anilinesa |
|
|
|
|
||||||||
|
|
|
|
|
R1 |
N |
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R7 |
|
|
R3 |
|
|
|
|
|
|
|
|
|
R6 |
|
|
R4 |
|
|
|
|
|
|
|
|
|
|
|
R5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
|
3 |
2 |
nN |
References |
|
|
|
|
|
|
|
|
8.07 |
|
9.20 |
|
10.80 |
26 |
Me |
|
|
|
|
|
|
7.68 |
|
9.10 |
|
10.28 |
26, 82 |
Et |
|
|
|
|
|
|
7.61 |
|
9.06 |
|
10.22 |
26 |
Pr |
|
|
|
|
|
|
7.53 |
|
9.00 |
|
10.11 |
26, 82 |
i-Pr |
|
|
|
|
|
|
7.51 |
|
8.98 |
|
10.09 |
26, 82 |
Bu |
|
|
|
|
|
|
7.55 |
|
9.03 |
|
10.10 |
26 |
t-Bu |
|
|
|
|
|
|
7.62 |
|
9.04 |
|
9.99 |
26 |
CH2Ph |
|
|
|
|
|
|
7.57, |
7.74 |
9.10 |
|
10.14 |
26, 82 |
Me |
Me |
|
|
|
|
|
7.45 |
|
9.00 |
|
9.85 |
42 |
Me |
Me |
Me |
|
|
|
Me |
7.85 |
|
8.60 |
|
8.85 |
42 |
Me |
Me |
|
|
Me |
|
|
7.48 |
|
9.06 |
|
9.65 |
70 |
Me |
Me |
|
|
CN |
|
|
7.86 |
|
9.56 |
|
10.19 |
81 |
Me |
Me |
|
|
COMe |
|
|
7.69 |
|
9.15 |
|
9.97 |
83 |
Me |
Me |
|
|
COCH2Cl |
|
|
7.69 |
|
9.24 |
|
10.06 |
83 |
Me |
Me |
|
|
COCH2Br |
|
|
7.7 |
|
9.2 |
|
10.1 |
83 |
|
|
Me |
|
|
|
|
7.84 |
|
8.84 |
|
10.63 |
75 |
|
|
Me |
|
|
|
Me |
7.75 |
|
8.50 |
|
10.55 |
42 |
|
|
|
Me |
|
|
|
7.82 |
|
8.89 |
|
10.55 |
75 |
|
|
|
|
Me |
|
|
7.81 |
|
9.06 |
|
10.50 |
75 |
Et |
Et |
|
|
|
|
|
7.20 |
|
8.90 |
|
9.70 |
42 |
Et |
Et |
|
|
CN |
|
|
7.65 |
|
9.45 |
|
10.08 |
81 |
Pr |
Pr |
|
|
|
|
|
7.12 |
|
8.90 |
|
9.63 |
26 |
i-Pr |
i-Pr |
|
|
|
|
|
7.17 |
|
7.81 |
|
8.93 |
26 |
Bu |
Bu |
|
|
|
|
|
7.00 |
|
8.76 |
|
9.52 |
26 |
(CH2)4 |
|
|
|
CN |
|
|
7.56 |
|
9.41 |
|
10.05 |
81 |
(CH2)5 |
|
NH2 |
|
CN |
|
|
7.89 |
|
9.64 |
|
10.10 |
81 |
|
|
|
|
|
|
7.69 |
|
8.59 |
|
10.55 |
84 |
|
|
|
|
|
|
|
|
|
|
|
|
11.01 |
|
|
|
|
NH2 |
|
|
|
7.60 |
|
8.26 |
|
10.05 |
84 |
|
|
|
|
|
|
|
|
|
|
|
11.44 |
|
|
|
|
|
NH2 |
|
|
7.34 |
|
9.10 |
|
9.71 |
84 |
|
|
|
|
|
|
|
|
|
|
|
11.54 |
|
|
|
F |
|
|
|
|
8.18 |
|
9.58 |
|
10.95 |
75 |
|
|
|
F |
|
|
|
8.32 |
|
9.30 |
|
10.96 |
75 |
|
|
|
|
F |
|
|
8.18 |
|
9.57 |
|
10.91 |
75 |
|
|
|
|
Cl |
|
|
8.18 |
|
9.51 |
|
10.64 |
70 |
|
|
Cl |
|
|
|
Cl |
8.20 |
|
9.10 |
|
11.10 |
42 |
|
|
F |
F |
F |
F |
F |
8.95 |
|
9.75 |
|
11.50 |
42, 71 |
Ph |
|
F |
F |
F |
F |
F |
8.17 |
|
9.54, 9.66 |
11.16 |
31 |
|
Ph |
Me3Si |
F |
F |
F |
F |
F |
8.05 |
|
9.29, 9.46 |
10.08 |
31 |
|
|
|
Ph |
|
|
|
|
7.70, |
8.70 |
9.15, |
9.50 |
10.60 |
85 |
SiMe3 |
|
|
|
|
|
|
7.70 |
|
9.01 |
|
10.09 |
31 |
SiMe3 |
SiMe3 |
|
|
|
|
|
8.25 |
|
9.03 |
|
10.43 |
26 |
a R1. . .R7 is H if not indicated otherwise

176 Paul Rademacher
relative to the benzene ring is rather small < ca 10° , in 7 and 10 it is about 20 30°. In accordance with a scheme involving an excited state crossing it was found that the rate constant for formation of the twisted intramolecular charge transfer excited state increases considerably with the ground state twist angle .
R R
N R N R N
CN |
CN |
CN |
R = Me, Et |
R = H, Me |
|
(6,7) |
(8,9) |
(10) |
Et |
|
|
N |
|
N |
CN |
CN |
(11) |
(12) |
2. Aminonaphthalenes
The PE spectra of ˛- and ˇ-naphthylamine (13,14) were studied by Maier86 and by Klasinc and coworkers87. Maier86 has also analysed the spectra of peri-amino and dimethylamino naphthalenes (15 19).
From the nodal properties of the naphthalene MOs (Figure 7) it is obvious that attachment of substituents in positions 1,4,5 and 8 will have only little effect on 5 whereas the other MOs will be affected. Substituents in the other positions will interact with all MOs of naphthalene in amounts proportional to the size of the coefficients in the respective positions.
The expectations regarding 4 are excellently confirmed in ˛-naphthylamine (13) (Figure 8). The IP( 4) value of this compound is practically the same as that of naphthalene while, for 3 and 5, IP values of ca 0.3 eV are found. However, alsoIP( 2) is very small. For ˇ-naphthylamine (14) 2 5 are destabilized in a much more uniform manner relative to naphthalene by 0.4 0.7 eV.
Similar statements for IP( 4) can be made for the two diaminonaphthalenes (15,16). Peri-substituted naphthalenes are examples of molecules with strong intramolecular crowding which can result in unique physicochemical properties. 1,8- Bis(dimethylamino)naphthalene (19) (‘proton sponge’88,89) has been found to have an

4. Photoelectron spectra of amines, nitroso and nitro compounds |
177 |
|||
|
NH2 |
|
|
NH2 |
|
|
|
NH2 |
|
|
|
|
NH2 |
|
(13) |
|
(14) |
(15) |
|
NH2 |
NH2 |
|
N |
N |
N
(16) |
(17) |
(18) |
N N
(19)
π5 |
π4 |
π3 |
D2h au |
b1u |
b2 g |
IP (eV) 8.15 |
8.88 |
9.98 |
π2 |
π1 |
b3 g |
b1u |
10.87 |
12.42 |
FIGURE 7. Nodal characteristics of the five occupied MOs of naphthalene

178 |
Paul Rademacher |
7
8
9
eV
10
11
12
n−
π(a2)
π(au)
π(b1) π(b1u) n+
n+
π(b2g) π(b1)
π(b1)
π(a2) π(b3g)
(CH3)2N N(CH3)2
π(au)
n−(bg)
π(au)
n+(au)
π(bg)
π(bg)
N(CH3)2
N(CH3)2
|
|
π(a2) |
π(au) |
|
|
|
|
|
π |
|
|
|
|
|
π |
|
π |
|
|
|
|
|
|
|||
|
|
π(b1) |
|
π(bg) |
|
|
π |
|
|
|
|
|
|
|
|||
n |
|
|
|
|
π |
|
|
|
|
|
|
|
π(au) |
|
|
|
|
π |
|
π(b1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
π |
|
π |
π |
|
π(a2) |
|
π(au) |
|
|
|
π |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
|
|
π(b1) |
|
|
π |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
π |
|
|
π(bg) |
|
|
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N(CH3)2 NH2 NH2 |
NH2 |
NH2 |
 NH2
NH2
NH2
FIGURE 8. Correlation diagram of the ionization potentials of aminonaphthalenes. Reproduced with permission from Reference 86
abnormally high basicity, pKa = 12.34, which has been associated with relief of steric strain and of nitrogen lone-pair interactions on protonation.
In 19 the first and the fourth IP are assigned to the two nN ionizations (nC and n ) while in 1,5-bis(dimethylamino)naphthalene (18) the corresponding ionizations are IP2 and IP4. The split IP D IP n IP nC is 2.0 eV in 19 and 0.82 eV in 18. It is remarkable that the larger split in 19 is not only caused by direct through-space interaction of the lonepairs but also by through-bond interactions with naphthalene orbitals86,90. The analysis of the PE spectra of 18 and 19 suggests that the dimethylamino groups are rotated by about 60°86 .
D. IP Values and Proton Affinities
It is well known that alkyl substitution changes the basicity of amines. However, solvation effects lead to an anomalous order of basicities in solution (NH3 ³ tertiary amine < primary amine < secondary amine). From gas-phase proton affinity data the intrinsic effects of alkyl substituents can be evaluated and a quite regular order (NH3 < primary amine < secondary amine < tertiary amine) is obtained91.
For simple aliphatic amines, like the methylamines, there is a linear inverse correlation between proton affinities and vertical IPs34. A low IP value should therefore indicate high proton affinity and vice versa. The proton affinity PA(B) of a molecule B is related to the homolytic bond energy D(BC H) in the conjugate acid, as indicated by equation 8. If the homolytic bond dissociation energy is assumed to be constant for a particular functional group, e.g. N H, the proton affinity will exhibit a linear correlation with the quantity
IP(H) IP(B), and such |
46,92 |
. |
|
nitrogen lone-pair ionizations |
|
|
|
PA(B) D IP(H) IP(B) C D(BC H) |
8 |
||
correlations have been reported for the proton affinities and the
