

7. NMR of compounds containing NH2, NO2 and NO groups |
315 |
Since the correlation of 17O chemical shifts of nitrobenzenes and nitrostyrenes is very good and nitrobenzene chemical shifts have been related to the -electron density on oxygen57, it appears that the chemical shift of the nitrostyrenes is dependent upon the
-electron density on the nitro oxygen also.
C. Nitroso Compounds
1. Substituent effects on 14,15N and 17O chemical shifts
C-nitroso. Following disagreements between earlier studies for nitrosobenzene59,60, Dahn and coworkers61 measured the υ 17O of several C-nitroso compounds (see Table 15) and confirmed very low field values in the region of 1530 1550 ppm, among the most shielded values reported so far59,62,63. Association to give azodioxy dimers led to shifts at much higher field (440 š 20 ppm).
Tautomerism exists in the case of o- and p-nitrosophenols and naphthols which exist mainly as the quinone oximes, and which also give high field shifts. The values for the oxime groups in the 1,2-naphthoquinones, for example, were 229 and 265 ppm for the 1-oxime and the 2-oxime respectively.
These high field values occur also in nitrones such as Ph N(O)DCH Ph (at 377 ppm) and pyridine N-oxide (349 ppm).
Nitrogen shifts in these compounds behave similarly (Table 16).
S-nitroso, N-nitroso and O-nitroso. Some nitrogen and 17O shift ranges for these compounds are listed below for comparison with the C-nitroso.
|
|
15N |
17O |
||
Nitrosamines |
R2N NDO |
200 |
|
155 |
670 |
|
|||||
Nitrites |
RO NDO |
207 |
|
180 |
800 |
|
|||||
Thionitrites |
RS NDO |
400 |
|
450 |
1300 |
|
|||||
Nitroso |
RC NDO |
500 |
|
590 |
1540 |
|
|||||
This concordance between the nitrogen and 17O shifts arises because of the importance of the E term, a rough measure of which is given by UV spectra and CD maxima. Some comparisons are given in Table 17.
The high shift values for NOC (474 ppm for 17O and 3 ppm for nitrogen) relative to lower values for X NDO compounds is good evidence for differences in chemical shift anisotropy as pertain in 13C NMR for the alkynes relative to alkanes and alkenes64. The higher symmetry of acetylene leads to a high field shift.
TABLE 15. 17O chemical shifts of C- and S-nitroso compounds61a
Compound |
υ(17O) (NDO) (ppm) |
Me3C NDO |
1538.2b |
Ph NDO |
1542.4 |
1533.4 |
|
2-Me C6H4 NDO |
1543.1 |
Ph3CS NDO |
1550.8 |
1293.0 |
|
AcNHCH(COOH) CMe2S NDO |
1312.4 |
a Solvent: CH3CN, relative to H2O.
b A high field peak at 390 ppm corresponds to the azodioxy dimer.
316 |
Edward W. Randall and Christiana A. Mitsopoulou |
|||||
|
|
TABLE 16. 15N chemical shifts of nitroso compounds |
||||
|
|
in CDCl3 measured with respect to 4.5M NH4NO3 |
|
|||
|
|
Compound |
Solvent |
υ15N (ppm) |
||
|
|
Ph NO |
none |
913 |
|
|
|
|
Me2N C6H4 NO |
CDCl3 |
804.3 |
|
|
|
|
Ph3C S NO |
CDCl3 |
806.5 |
|
|
|
|
Ph2N NO |
none |
268.7 (N-1) |
||
|
|
PhMeN NO |
|
554.4 |
(N-2) |
|
|
|
none |
250.7 (N-1) |
|||
|
|
|
|
543.8 |
(N-2) |
|
|
|
|
|
|
|
|
δ(17O) (XNO) (ppm)
TABLE 17. |
14,15N and 17O chemical shifts, CD maxima and longest wavelength |
|||
UV/v is absorptions, and rotational barriers of nitrosyl compounds66 |
|
|||
|
|
|
|
Rotational |
|
|
|
n ! Ł (nm) |
barrier |
Compound |
υ14,15N |
υ17O |
(kcal mol 1) |
|
RR0 R00 CNO |
590 |
1538 |
680 |
|
PhNO |
530 |
1533 |
755 |
7.7 |
RSNO |
452 |
1300 |
600 |
|
RONO |
190 |
800 |
355 |
10.5 |
R2NNO |
155 |
670 |
365 |
23 |
O NO |
229 |
650 |
357 |
|
NOC |
3 |
474 |
<210 |
|
1600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me3C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|||
|
|
|
|
|
|
|
|
|
|
|||
1400 |
|
|
|
|
|
|
|
|
|
|
|
|
1200 |
|
|
|
|
|
RS |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
1000 |
|
|
|
|
|
|
|
|
|
|
|
|
800 |
RO |
|
|
Cl |
||||||||
|
|
|||||||||||
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
R2N |
|||||||||
|
|
|
||||||||||
O− |
|
|
||||||||||
600 |
|
|
||||||||||
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
300 |
400 |
500 |
600 |
700 |
800 |
|
|
|
λ(nm) |
|
|
FIGURE 4. Plot of υ17O) vs CD maxima and n ! Ł of nitroso compounds X NO. Reproduced from Reference 61 by permission of Verlag Helvetica Chimica Acta AF

7. NMR of compounds containing NH2, NO2 and NO groups |
317 |
Donor groups X conjugated with NO in XNO (X D Me < Ph < RS < RO < R2N < O ) increase the shieldings at the nitroso O-atom via resonance X NDO $ XC DN O . The resonance-donating power of the group X can be represented by Taft’s substituent constant R. Figure 4 shows that the plot of υ(17O) vs R is reasonably linear (r D 0.95).
The large difference between the nitrogen shieldings in nitroso groups and those molecules that are isomeric, tautomeric or related in any way to nitroso structures facilitates their spectral distinction1b.
The amino nitrogens of N-nitroso compounds are deshielded substantially more than those of electronically analogous amides, and well separated resonances frequently can be detected65, where geometrical isomerism is possible.
Substituent effects have been discussed in terms of electronic and geometrical influences on the extent of nitrogen lone-pair delocalization66.
V.NMR DETERMINATIONS OF STRUCTURE IN SOLUTION A. Substituent Effects on One-bond 15N 13C Couplings
In aniline derivatives, ring substituents are known to have to profound influence on the directly bonded 15N 1H and 15N 13C spin coupling interactions67 69. Binsch and coworkers70 have successfully related the one-bond 15N 13C coupling constant to the atomic hybrizations by the empirical equation 4
1J 15N13C D SNSC/80 |
4 |
where SN and SC represent the percentage s character of the N and C contributions to the N C bond. This relationship works reasonably well provided that the coupling interaction is dominated by the contact mechanism71 73 and the s electron character of the lone pairs remains unchanged74. Thus, in going from aniline to p-nitroaniline, the observed increases in 1J(15NH) and 1J(15N13C) are readily explained in terms of a substituentinduced delocalization of the lone pair corresponding to a change in hybridization to give a more flattened amino-nitrogen pyramid.
The effect on 15N and 13C chemical shifts in anilines75 77 and nitrobenzenes74 of a reduced resonance interaction caused by ortho substituents which sterically twist the amino and nitro groups from coplanarity with the aryl ring has been extensively investigated and is fairly well documented.
The effect on 1J(15N13C) of steric inhibition of conjugation has been studied90 in the case of a series of 15N-labelled nitrobenzenes and a series of N,N-dimethylanilines (Table 18).
In the case of nitrobenzenes, the narrow range of the observed 1J(15N13C) values reported in Table 18, approximately 2 Hz, despite the substantial changes in the electronic character of the substituents involved, suggests that the N C p-bond order is probably very small and is not of primary importance in determining the coupling in these systems. Also, it seems that the nominally sp2 hybridization of the nitro-group nitrogen, and the aryl carbon to which it is coupled, remain essentially unchanged.
In the case of anilines several points are noteworthy of consideration in Table 18.
It may be seen that enhanced coupling results from both methylation at nitrogen (1.1 Hz) and the introduction of amino group at the para position (2.6 Hz), whereas methylation at the ortho positions causes a small decrease (0.2 Hz). The decrease in 1J(15N13C) of N,N-dimethylaniline and its N,N-dimethyl derivatives is explained by inhibition of the resonance interaction resulting from sterically induced twisting of the substituent group from coplanarity with the aryl ring.
318 |
Edward W. Randall and Christiana A. Mitsopoulou |
|
||||||
|
TABLE 18. Some experimental 1J(15N13C) values in nitrobenzene and aniline derivatives78 |
|||||||
|
Compound |
|
X |
Y |
Y0 |
Z |
Z0 |
Jexpa (Hz) |
|
|
|
H |
H |
H |
H |
NH2 |
14.8 |
|
|
|
Me |
NO2 |
H |
Me |
NH2 |
15.8 |
|
Y′ |
Z′ |
NH2 |
H |
H |
H |
H |
16.2 |
|
|
|
|
|
|
|
||
X |
15NO2 |
NH2 |
Me |
Me |
H |
H |
15.8 |
|
|
Y′ |
Z |
Me |
H |
NO2 |
Me |
NH2 |
15.6 |
|
|
|
||||||
|
|
|
MeO |
H |
Me |
H |
H |
15.9 |
|
|
|
NH2 |
H |
H |
Me |
Me |
16.6 |
|
|
|
Me2N |
Me |
NO2 |
H |
H |
14.7 |
|
|
|
t-Bu |
H |
H |
H |
H |
15.0 |
|
|
|
Me |
NO2 |
H |
H |
H |
18.0 |
|
|
|
Me |
H |
H |
H |
H |
14.9 |
|
|
|
t-Bu |
H |
H |
t-Bu |
t-Bu |
13.7 |
|
|
|
Cl |
H |
H |
H |
H |
15.4 |
|
|
|
H |
H |
H |
H |
H |
14.7 |
|
|
|
H |
NO2 |
H |
H |
H |
15.9 |
|
|
|
Me |
H |
H |
Me |
Me |
14.7 |
|
|
|
NO2 |
H |
H |
H |
H |
15.3 |
|
|
|
H |
H |
|
H |
|
12.1 |
|
|
|
NO2 |
H |
|
H |
|
14.7 |
|
|
|
H |
H |
|
Me |
|
13.2 |
|
|
Y |
NO2 |
H |
|
Me |
|
15.6 |
|
|
|
|
|
|
|
|
|
|
|
|
Z |
|
|
|
|
|
X |
15N |
H |
Me |
|
Me |
|
12.9 |
|
|
|
|
Z |
|
|
|
|
|
|
|
Y |
NO2 |
Me |
|
Me |
|
14.2 |
|
|
|
|
|
||||
|
|
|
H |
Me |
|
H |
|
11.9 |
|
|
|
NO2 |
Me |
|
H |
|
14.8 |
|
|
|
Br |
Br |
|
H |
|
17.6 |
|
|
|
Br |
Br |
|
Me |
|
16.3 |
a 1J(15N13C) values and 13C chemical shifts were measured at 5000 Hz/16 K real points.
B. One-bond 15N 1H Couplings
It is well known that one can obtain valuable structural and stereochemical information from 1J(15N 1H) and study the distribution of bonding electrons. The theory of spin spin coupling is covered satisfactorily in many books79,80.
Values of one-bond coupling 1J(15N 1H) are the largest in magnitude of all 15N couplings to the proton and are generally regarded as being dominated by the Fermi contact term.
The possibility of obtaining spin spin coupling information from measurements involving the 14N nucleus is generally impossible, because rapid quadrupolar relaxation of 14N

7. NMR of compounds containing NH2, NO2 and NO groups |
319 |
causes decoupling of the spins. In the symmetrical cases for which results are available, 14N spin couplings to a nucleus X may be converted to 15N couplings by equation 5, simply by use of the ratio of the s, since there are no other isotope effects arising from electronic changes.
J15N |
|
X D 1.4027J14N |
|
X |
5 |
|
|
One-bond 15N couplings to 1H are negative (because of the negative sign for the magnetogyric ratio) (Table 19) and can vary from about 50 to around 135 Hz in magnitude for different compounds (Table 20).
There is a correlation between the amount of s character in the N H bond and the magnitude of the corresponding spin spin coupling81:
%s D 0.341J15N |
|
1H |
6 |
|
so that the magnitude grows with increasing s-character of the bond concerned, with the main exception of the ketimines. Susskind and coworkers82 carried out Partially Restricted Molecular Orbital (PRMO) calculations at the INDO level of approximation in order to study s- and p-transmitted components for 15N 1H couplings in a set of molecules. As expected the values of one-bond couplings are transmitted almost exclusively through the s-framework. The only contribution to the 15N 1H coupling is the Fermi contact term. Table 21 contains some data for the coupling between 15N and 1H.
TABLE 19. Some 15N |
|
1H couplings across one bond |
|
|||
|
|
|||||
Compound |
|
Solvent |
1J(15N |
|
1H) (Hz) |
Reference |
|
|
|||||
PhNDNC6H4NH2-p |
|
DMSO |
87.0 |
85 |
||
4-NH2C6F4NH2 |
|
CH2Cl2 |
80.3 |
86 |
||
4-MeOC6F4NH2 |
|
CH2Cl2 |
85.0 |
86 |
||
4-MeC6F4NH2 |
|
CH2Cl2 |
80.3 |
86 |
||
4-CF3F4NH2 |
|
CH2Cl2 |
90.6 |
86 |
||
4-CNC6F4NH2 |
|
CH2Cl2 |
87.9 |
86 |
||
4-NO2C6F4NH2 |
|
CH2Cl2 |
90.4 |
86 |
||
2-NO2C6F4NH2 |
|
CH2Cl2 |
90.4 |
86 |
||
3-NO2C6F4NH2 |
|
acetone-d6 |
85.4 |
87 |
||
4-NO2C6F4NH2 |
|
acetone-d6 |
91.0 |
87 |
||
PhNH LiC |
|
acetone-d6 |
54.7 |
88 |
||
4-Aminoazobenzene |
|
Me2SO-d6 |
88.2 |
84 |
||
TABLE 20. Values of one-bond 15N couplings to 1H
Compounds |
Range of 1J(15N |
|
1H) (Hz) |
|
|||
Ketimines, R2CDNH |
50 |
|
|
NH in three-membered rings |
50 to 65 |
||
Alkylamines, ammonium ions, hydroxylamines, |
60 to 80 |
||
hydrazines |
|
|
|
Arylamines and enamines |
80 to 95 |
||
NHC in cations derived from aromatic azine systems |
90 |
|
|
and from imines |
|
|
|
Amides and related structure |
90 to 100 |
||
NH moieties in aromatic azole systems |
95 to 110 |
||
Protonated nitriles, R C NH |
135 |
|
|
320 |
Edward W. Randall and Christiana A. Mitsopoulou |
|||||||
|
TABLE 21. Transmission mechanisms of one-bond 15N |
|
1H couplings82 |
|||||
|
|
|||||||
|
|
|
|
Fermi contact |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecule |
|
INDO |
s |
p |
|
Experimental (Hz) |
|
|
|
|
|
|
|
|
||
|
Cyanamide |
85.81 |
85.57 |
0.24 |
|
89.4 |
||
|
Methylenimine |
3.75 |
0.94 |
2.81 |
|
|
|
|
|
Pyrrole |
88.41 |
86.86 |
1.54 |
|
96.48 |
||
|
Aniline |
90.56 |
89.56 |
1.00 |
|
92.0 |
||
|
Formamide |
78.68 |
77.69 |
0.99 |
|
88.0 |
||
The determination of the structures of anilines from the one-bond 15N H couplings is a typical example. The lone pair of nitrogen has more p character than in NH3 or the ammonium ion83 because it is partially delocalized into the p system. This leads to an increase of the s character in the N H bond and thus an increase in the comparison to the values for NH3 (J D 61.2 Hz, liquid), aniline (J D 82.3 Hz, DMSO) and p- nitroaniline (J D 89.4 Hz, DMSO). The major structural factor is the increase in the HNH bond angle on going from NH3 to aniline and nitroaniline. The one-bond 15N 1H coupling constants in anilines have been found to be intermediate between those expected for tetrahedral nitrogen (ammonium ion, sp3) and trigonal nitrogen (pyrolle, sp2) which are 73.5 and 96.5 Hz, respectively.
Other structural studies84 involving the use of 1J(15N 1H) data include evidence for the site of protonation of 4-aminoazobenzene (10).
N N |
NH2 |
(10)
4-aminoazobenzene has three different kinds of nitrogen atoms within one molecule. One is an amino nitrogen (N ) (with an approximately p-hybridized lone pair), and the others are azo nitrogens (N˛, Nˇ) with s-hybridized lone pairs. Upon protonation, the hybridization state of the lone pairs and the electronic environment around the nitrogen atom are greatly affected, resulting in marked changes in sign and magnitude of spin spin coupling constants and chemical shifts of 15N nuclei together with changes in the nuclear Overhauser effect (NOE) of the 15N nucleus. It has been confirmed84 that in weak acid solutions there are two monocationic species, in one of which the proton is attached to the ˇ-azo nitrogen and in the other to the amino nitrogen, and that in fairly strongly acidic solutions the 4-aminoazobenzene molecule exists as a dicationic species protonated at the amino and ˛-azo nitrogens but not at the ˇ-azo nitrogen.
C. Liquid Crystal Solvents
The advantages of the study of the NMR spectroscopy of solutes dissolved in liquid crystal solvents for structural studies have been exploited extensively despite the wellknown limitations of the technique, and many compounds have been studied89,90.
The 1H magnetic resonance spectra of pyrrole- 15N in two nematic solvents have been reported91. The dipolar coupling constants were used to derive relative internuclear distances, and it was found that the N H bond distance relative to the distance

7. NMR of compounds containing NH2, NO2 and NO groups |
321 |
between the ˇ-protons varied by almost 2% for the five solutions examined. Two liquid crystals were used as solvents: N(-p-ethoxybenzylidene)-p0-n-butylaniline (EBBA) which forms a nematic mesophase in the temperature range 35 79 °C, and p-n-butyl-p0- methoxyazoxybenzene (Phase IV), which has a nematic range of 16 76 °C.
It was shown that the solute molecules orient with the two-fold axis perpendicular to the liquid-crystal optic axis. This effect may be caused by the orienting influence of hydrogen bonding between the NH proton of pyrrole-15N and the solvent the calculated NH bond length is indeed slightly different from the value determined by microwave spectroscopy and differs in different nematic solvents (on the assumption that the anisotropy in the onebond J-coupling is negligible). The dipolar couplings were re-analysed92 by inclusion of the effect of harmonic vibrational motion. The apparent NH bond length is then reduced by up to 3%, and the total spread in value is reduced from 2 to 1%. This leads to the conclusion that there is a real difference of about 3% between the N H bond lengths for pyrrole in Phase IV and in the gas.
The NMR spectra of p-nitroaniline[1-14N], p-nitroaniline[1-15N] in Merck’s nematic Phase VIIa and p-bromoaniline[1-15N] in nematic Phase IV, each partially oriented in the solvent, have been studied93. At first the difficulties experienced with aniline and its derivatives90, namely broad 1H lines, were attributed to the quadrupole effect of the 14N nucleus94. But it was been shown93 that although the quadrupole effect of the 14N nucleus is not negligible, the main reason for line broadening is the effect of water-catalysed exchange of the NH2 hydrogens, since suppression of exchange allows reasonably narrowlined spectra of p-nitroaniline[1-14N] to be obtained. The use of 15N-enriched samples, besides producing narrower lines, provides a sufficient number of dipolar couplings for the detailed determination of the molecular shape.
The molecular reorientation is found to be correlated with NH2 internal motions. The non-planar nature of the molecules is shown by both the uncorrected and the vibrationally corrected data.
Considerable changes are produced by the substituents in all the parameters involving the NH2 group: the N H distances increase markedly; the H N H angles decrease toward the values for ammonium ion (109.5°) and for ammonia (106.7°), and the dihedral out-of-plane angles increase slightly (by 5° for p-nitroaniline[1-15N], but by very little for p-bromoaniline[1-15N].
Diagonalization of the ordering matrix93 leads to the determination of the angle between its principal axis and the molecular fixed axis. The result is 6.5 š 0.3° for p-nitroaniline and 3.2 š 0.5° for p-bromoaniline.
The effect of the substituents at the NH2 pyramid is confirmed to be a flattening by electron-withdrawing substituents, and an accentuation by electron donors since the order observed for the out-of-plane angle, ϕ, with respect to the substituent R is
NO2 < H Br < F
D. Solid State NMR
The last ten years have seen increasing development in the field of high-resolution solid state NMR. This is due to the exploitation of relatively new methodologies, such as those which enable better resolved spectra to be obtained from quadrupolar nuclei and those which facilitate the measurement of internuclear distances.
There are also reports of interesting J coupling effects in some magic angle spinning (MAS) spectra as well as new experiments for purposes such as spectral editing.
So far, there have been rather few applications of 15N CPMAS NMR at the natural abundance of the isotope95 and, in most cases, 15N-labelling has been employed.

322 Edward W. Randall and Christiana A. Mitsopoulou
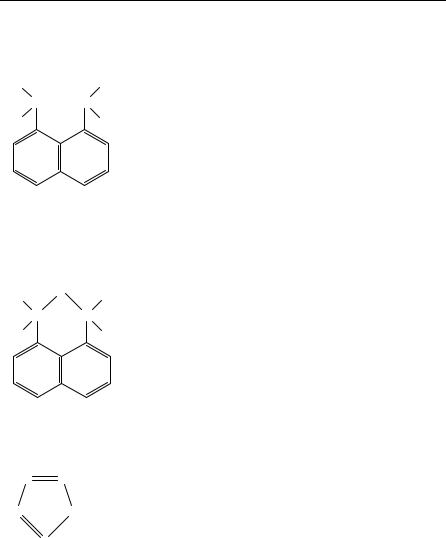
As a typical example we give the results of 13C and 15N NMR studies on 1,8- bis(dimethylamino)naphthalene and its monohydrated salt with tetrazole96 (Table 22).
On the basis of the solution and solid state 15N results, an equilibrium involving the half protonated form of the base in the solution of the complex salt has been identified. The 13C results for the solutions, on the other hand, are very similar to those found for the solid state, and are rather insensitive to salt formation. So it seems that the 15N NMR data provide the most suitable means of investigating the structure of the compounds studied in both solution and the solid state.
In 1989 Gullion and Schaefer97 described the rotational-echo, double-resonance (REDOR) technique. Distances measured by this method may be used in the determination of bound-ligand conformations without the limitations imposed by crystallization, molecular weight or solubility. They have performed98 REDOR 15N 13C NMR experiments, on an alanine cocrystallized from five-component alanines, isotopically enriched in 13C, 15N or 12C. REDOR 15N 13C NMR involves the dephasing of 13C magnetization by 15N 180° pulses synchronized with magic angle spinning. The C N dipolar coupling determines the extent of dephasing. The results of these experiments on alanine show that it is practical to use REDOR to measure the C N dipolar coupling in 5 mmol of a 13C 15N labelled pair having an internuclear separation of the order of 4.5 A˚ .
Garbow and McWherter99 have defined the methods and requirements for constructing structural models from REDOR data, using the tripeptide melanostanin [Pro-Leu-Gly- NH2] as a model system. REDOR experiments were performed on a series of selectively 13C- and 15N-labelled melanostatins allowing the distances between the labelled carbon and nitrogen atom in each case to be determined. A comparison with the X-ray structure showed excellent agreement. The REDOR distances provided effective constraints on the values of the backbone dihedral angles of melanostatin, therefore providing a direct determination of the solid state conformation. The determination of distances in a large molecule like a protein, by using DANTE-selected Rotational-Echo Double-Resonance NMR100, has been proposed.
An interesting ‘three-dimensional separated-local-field/dilute-spin-exchange solid state NMR experiment’ has been reported101 where homonuclear spin-exchange cross-peaks provide correlation in the ω2/ω3 plane. Resonances are resolved along the ω1 axis by their heteronuclear dipolar couplings. The experiment was demonstrated experimentally with the 1H 15N heteronuclear and 15N 15N homonuclear dipole dipole interactions in a single crystal sample of uniformly 15N-labelled glycylglycine at an arbitrary orientation. Although glycylglycine has two magnetically inequivalent molecules per unit cell giving rise to four nitrogen resonances, the resonances from the two amino acid groups in the unit overlapped accidentally at this orientation of the crystal. In the three-dimensional experiment, however, the resonances were nevertheless resolved by their heteronuclear dipole dipole couplings. This type of experiment is expected to be particularly important for studying proteins in the solid state, because it directly addresses the severe overlap and assignment problems for both chemical shifts and dipolar interaction and, as a bonus, provides a clear way to measure the magnitudes of the splittings from heteronuclear dipole dipole interactions.
The conformational properties of mono-substituted cyclohexanes, C6H11X, in their thiourea inclusion compounds have been studied102. Variable-temperature MAS spectra demonstrate that a ‘chair-chair’ ring inversion process occurs in the thiourea tunnel, in which the axial and equatorial conformers are interconverted. Predominance of the equatorial conformer is found when X D NH2.
Solid state 15N NMR has also been used103 to study lyophilized powders of 15N- enriched histidine and imidazole, prepared from aqueous solutions with pH ranging
7. NMR of compounds containing NH2, NO2 and NO groups |
323 |
TABLE 22. 13C and 15N solution and solid state NMR data for 1,8-bis(dimethylamino)napthalene and its monohydrated salt with tetrazole96
|
|
Chemical shiftsa |
|
|
|
for a solution in |
CPMAS |
Compounds |
|
CD3CN |
chemical shiftsa |
|
N Me |
44.7 (134.4)b |
42.0 |
|
|
|
45.4 |
H3 C |
CH3 |
|
|
N |
N |
|
|
H3 C 8
7
6
4a
5
H+
H3 C
N
H3 C 8
7
6
4a
5
3′N N 2′
1 CH3
2
3
4
CH3
N
1 CH3
2
3
4
C2 |
113.8 (156.9) |
113.4 |
C1a |
121.3 |
|
122.1 |
C4 |
122.5 |
|
122.1 |
C3 |
126.5 |
(159.1) |
125.3 |
C4a |
138.8 |
(160.8) |
138.2 |
C1 |
151.7 |
|
150.6 |
N |
338.1 |
b |
329.7 |
N Me |
46.0 |
(138.4) |
44.7 |
C2 |
119.3 (161.7) |
117.8 |
C1a |
120.5 |
|
124.3 |
C4 |
127.3 |
(162.5) |
128.7 |
C3 |
127.5 |
(162.7) |
131.2 |
C4a |
137.2 |
|
136.2 |
C1 |
147.6 |
|
151.0 |
4′ |
− |
H2 O |
N |
343.7 |
|
340.0 |
||
N |
N 1′ |
|
|
|
|
|
|
|
|
|
|
C50 |
147.7 |
(204.0) |
145.2 |
||
|
|
|
N0 |
78.8 |
|
|
56.4 |
|
|
|
|
1 |
|
1.6 |
|
11.4 |
|
|
|
|
N0 |
|
|
|
||
|
|
|
2 |
|
|
|
|
|
a13 C chemical shifts are given with respect to TMS and 15N chemical shifts with respect to external neat nitromethane. b1 J coupling data are given in parentheses
324 |
Edward W. Randall and Christiana A. Mitsopoulou |
between 2 and 12.5. The 15N shift anisotropy is found to double on deprotonation of the -nitrogen atom and is dependent on the protonation state of nitrogen. The tautomeric exchange (11) is slow or non-existent in the solid as shown by the observation of two 15N resonances for the free base imidazole (R D H).
R |
|
|
R |
π N |
NH τ |
NH |
N |
(11)
Solid samples of histidine, prepared from solutions of different pH, show slow exchange between cationic and neutral forms. Furthermore, only a single tautomer (the N H) is observed for the neutral and anionic species.
The combined use of natural-abundance solid state 13C and 15N NMR has shown104 that the structure of the trimethoprim:sulphamethoxazole complex (TMP:SMZ) is best described by transfer of the proton N(7) of SMZ (12) to N(1) of TMP (13).
|
2 |
|
|
13 |
12 |
|
1O |
N 3 |
NH |
|
8 |
|
|
|
7 |
SO2 |
|
NH2 |
||
|
|
|
|
|
||
5 |
|
|
|
|
|
11 |
4 |
|
|
|
|
|
|
6 CH3 |
|
|
9 |
10 |
|
|
|
|
|
|
|||
|
|
|
(12) |
|
|
|
|
8 |
|
|
|
21 |
|
|
NH2 |
9 |
|
15 |
CH3 |
|
|
|
|
O |
|
||
3 N |
|
CH2 10 |
14 |
20 |
||
5 |
|
|
|
|
||
|
|
|
|
|
|
|
7 2 |
6 |
|
11 |
|
|
CH3 |
H2 N |
N |
|
|
13 |
O 19 |
|
|
1 |
|
|
12 |
18 |
|
|
|
|
|
|||
O
16 CH3
17
(13)
A solution-state and solid-state nuclear magnetic resonance study of the complex and its separate components in both their neutral and ionized (TMP hydrochloride and SMZ sodium salt) forms was undertaken in order to elucidate the TMP SMZ interactions. Inspection of the data for the complex in the solid state shows that the 13C chemical shifts are consistent with the ionic structure proposed by Nakai and coworkers105 (14). Stabilization of the complex is achieved by the resulting ionic interaction and by the formation of two intermolecular hydrogen bonds.
The results of a comprehensive 13C CP/MAS NMR study of the structure of solid polypeptides, prepared by polymerization of amino acid N-carboxyanhydrides under various conditions, have been reported105,107. In the case of poly(L-alanine) it was found that
