

7. NMR of compounds containing NH2, NO2 and NO groups |
305 |
para substituent exerts an effect on the resonance position that is similar to that observed for the anilines31,32.
A substantial divergence from the behaviour described up to now occurs with the 2,6-N,N-tetramethyl anilines (Table 7). The effect of the para substituent is no longer systematic, and no good correlation with substituent electronic properties is displayed. Evidently, the extent of nitrogen lone-pair delocalization in these compounds reflects a balance between electronic demand for, and steric inhibition of, delocalization that varies with the individual compounds. Nitrogen chemical shifts are a sensitive probe for these effects.
15N substituent chemical shifts (SCS) of a variety of anticonvulsant phenylacetanilides 1 and 2, with a substituent at the para or meta position of the aniline moiety, were analysed by means of DSP (dual substituent parameter) equations33.
The two parameters are and and appear in equation 1 or 2:
PhCH2 CONH |
X PhCH2 CONH |
H2 N |
X |
|
|
X |
|
(1) |
(2) |
|
(3) |
or |
15N SCS D I I C R R |
|
(1) |
15N SCS D I I C R R C C |
|
2 |
|
|
|
where I indicates inductive, R represents resonance, and C is a constant term specified for each correlation.
The 15N chemical shifts of 1 and 2 together with the data for the relevant para- substituted anilines (3) are summarized in Table 8. At first glance it is clear that the
TABLE 8. 15N chemical shiftsb (υN) and substituent chemical shifts (SCS, in parentheses) of phenylacetanilides 1 and 233
Substituent |
Series (1) |
Series (2) |
|||||
|
|
|
|
|
|
|
|
NH |
2 |
|
|
136.1 |
( |
C |
0.5) |
|
|
|
62.2a |
|
|
||
NHMe |
|
|
136.4 |
( |
C |
0.8) |
|
|
|
|
|
56.0a |
|
|
|
NMe2 |
|
|
136.0 |
( |
C |
0.4) |
|
|
( 2.3) |
42.3a |
|
|
|||
OH |
|
133.3 |
135.9 |
( 0.3) |
|||
OMe |
133.5 ( 2.1) |
|
( 0.3) |
||||
Et |
|
134.7 |
( 0.9) |
135.5 |
|||
Me |
|
134.9 |
( 0.7) |
135.3 |
( 0.3) |
||
H |
|
135.6 |
( 1.1) |
135.6 |
( 0.5) |
||
F |
|
134.5 |
135.5 |
||||
Cl |
|
134.5 |
( 1.1) |
135.1 |
( 0.5) |
||
Br |
|
134.7 |
( 0.9) |
|
( 0.6) |
||
CN |
|
137.6 |
(C2.0) |
134.9 |
|||
Ac |
|
137.5 |
(C1.9) |
134.8 |
( 0.8) |
||
NO2 |
138.2 |
(C2.6) |
135.2 |
( 0.4) |
|||
a Nitrogen of the substituent
bSolvent CD3 2SO, referred to NH3.

306 Edward W. Randall and Christiana A. Mitsopoulou
range of 15N SCN for 1 is small, and the chemical shift difference between the p-OH derivative (with a representative electron donor substituent) and the p-NO2 derivative (a representative electron acceptor) is only about 5 ppm, compared with a difference of some 25 ppm between the same two substituents reported for 15N (SCS) of para-substituted anilines (3).
It seems that 15N chemical shifts of amide nitrogen are much less sensitive to electron withdrawal and donation than 15N shifts of amines because of the strong conjugation of the amide nitrogen lone-pair electrons with the carbonyl groups, which compete for conjugation with the -systems of the benzene rings.
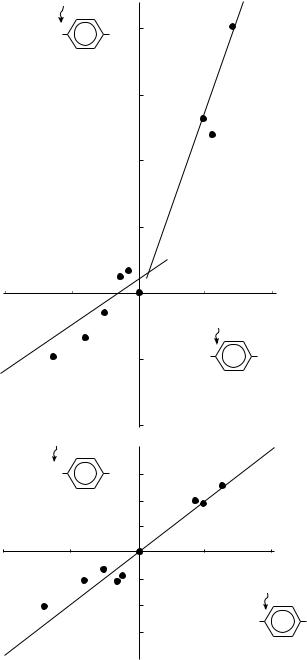
A plot of 13C SCS for C1 (i.e. carbon nuclei para to the substituent) of 3 against 15N SCS of 3 (Figure 1a) indicates that the slope changes as the substituents change from a donor to an acceptor to show the bilinear dependency. This is evidence for the presence of an interaction between the substituent and the NH2 group, a well-known phenomenon. For compounds (1) which carry such mild substituents as the NH(CDO) group rather than NH2, a rough linear correlation is expected between 15N shifts and 13C shifts of C1. The plot is shown in Figure 1b. The linearity is good, to indicate further a sharp contrast between a strong substituent NH2 and a mild substituent NH(CDO) .
The differences between 1 and 3 are revealed also in the DSP-NCR analyses of C1
shifts on these compounds34. The analyses were based on equation 3, namely |
|
13C SCN D I I C R R/1 εR |
3 |
where ε is a constant for each fixed substituent and characterizes the electron demand exerted by the fixed substituent on the resonance effects of the variable substituents.
The similarity observed for the correlation of the carbon shifts para to the substituent for 1 and 2 indicates that the nitrogen shift is a better probe of the transmission of the substituent effect to nitrogen. This conclusion can also be reached from a study of the polyfluorinated anilines.
According to the data of Table 9, the 15N signals of nitrogen in polyfluorinated anilines are shifted ca 29 ppm upfield with respect to their hydrocarbon analogues. As in the case of hydrocarbon compounds, the introduction of electron-accepting substituents in the fluorinated benzene ring leads to a downfield shift of the 15N signal of the amino group, the effect of ortho substituents being stronger than that of substituents in para position. Electron-donating substituents show an analogous effect which has the same sign, but the value of the shift is smaller (Table 9).
TABLE 9. 15N chemical shifts of nitrogen-containing polyfluoroaromatic compounds38.1 (ppm vs NH3)
Compound |
υN (ppm) |
Compound |
υN (ppm) |
4-NEt2C6F4NH2 |
30.0 |
2,6-F2C6F3NH2 |
26.6 |
4-NH2C6F4NH2 |
25.5 |
C6F5NMe2 |
16.7 |
4-MeOC6F4NH2 |
28.8 |
C6F4NCl2 |
104.2 |
4-MeC6F4NH2 |
43.2 |
4-CF3C6F4NCl2 |
95.1 |
4-HC6F4NH2 |
29.9 |
4-H-C6F4NO2 |
350.4 |
C6F5NH2 |
24.4 |
C6F5NO2 |
350.4 |
4-CF3C6F4NH2 |
44.4 |
4-CF3C6F4NO2 |
350.2 |
4-CNC6F4NH2 |
59.7 |
4-CH3OC6F4NO2 |
354.8 |
4-NO2C6F4NH2 |
51 |
4-NO2C6F4NO2 |
349.2 |
2-NO2C6F4NH2 |
54 |
2.6-F2C6F4NO2 |
354.3 |
2-NH2C6F4NH2 |
31.6 |
C6F5NO |
893.3 |
2-NO2-5-CF3C6F4NH2 |
52 |
C6F5CONH2 |
113.2 |
7. NMR of compounds containing NH2, NO2 and NO groups |
307 |
||||||
|
H2N |
X |
20 |
|
NO2 |
|
|
|
|
|
|
|
|
||
|
|
|
15 |
|
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
|
|
Ac |
|
|
|
|
N SCS |
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
|
5 |
|
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Br |
|
|
|
|
|
|
Cl |
|
|
|
|
|
−10 |
−5 |
|
H |
5 |
|
10 |
|
|
|
Me |
|
Cl SCS |
|
|
|
|
|
|
|
|
|
|
|
|
|
F |
|
|
|
|
|
|
OMe |
|
−5 |
H2N |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
−10 |
|
|
|
|
PhCH2CONH |
X |
3 |
|
|
|
|
|
|
|
|
2 |
CN |
NO2 |
|
|
|
|
|
|
|
|
||
|
|
N SCS |
|
|
Ac |
|
|
|
|
1 |
|
(b) |
|
|
|
|
|
15 |
|
|
|
|
|
−10 |
−5 |
Me |
H |
5 |
|
10 |
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F |
Cl |
Cl SCS |
|
|
|
|
|
|
|
|
|
|
||
|
OMe |
|
−2 |
|
|
|
|
|
|
|
−3 |
PhCH2CONH |
X |
|
|
|
|
|
|
|
|
|
|
FIGURE 1. Plots of 15N SCS against 13C C1 SCS for p-substituted: (a) anilines, (b) phenylacetanilides33
308 Edward W. Randall and Christiana A. Mitsopoulou
In the 15N NMR spectrum of pentafluorodimethylaniline, the 15N signal is shifted upfield with respect to the same signal in the 15N NMR spectrum of pentafluoroaniline. The analogous shift has been observed also earlier for hydrocarbon analogues35, and was attributed to the increased electron density on nitrogen in dimethylaniline due to weakening of the -character of the C N bond, reducing the paramagnetic contribution to the chemical shift value. In the case of substituents at nitrogen capable of strong p p interaction with it, the latter may be expected to be considerably deshielded and the corresponding signal in 15N NMR spectrum of N,N-dichloropolyfluoroanilines will be observed in a lower field with respect to that of pentafluoraniline36.
Summarizing, changes in screening of nitrogen induced by introduction of fluorine into nitrogen-containing aromatic compounds are said to be controlled mainly by the radial factor hr 3i2p N , which depends on the electron density on nitrogen37. When the pentafluorophenyl group and nitrogen, both strong electron acceptors, are directly bonded with each other, they act jointly, leading to increase of electron density on the RN fragment with respect to the hydrocarbon analogue, decreasing it on other fragments of the molecule. Increased electron density on nitrogen of polyfluorinated derivatives in comparison with their hydrocarbon analogues shows itself in the 15N NMR spectra as an upfield shift of the signal.
2. Substituent solvation effect on 15N anilines
A study39 of substituent effects on the 15N chemical shift (υ15N) (Table 10) for 4- substituted anilines in DMSO was interpreted in terms of substituent solvation-assisted resonance (SSAR) effects. Solvation of certain conjugated -electron-acceptor (CR) substituents has been found to give significant enhancements in the acidities of anilines, phenols and other acids40,41, and the magnitudes of these enhancements increase with
TABLE 10. 15N NMR chemical shifts of 4- substituted anilines39,42
X |
|
NH2 |
|
|
|
X |
υ15Na |
υ15Na |
OMe |
62.9 |
58.4 |
Me |
59.9 |
55.2 |
F |
60.7 |
55.8 |
Cl |
57.5 |
52.9 |
CF3 |
52.1 |
45.7 |
SCF3 |
51.3 |
45.2 |
H |
57.8 |
53.0 |
CO2Me |
49.1 |
41.9 |
CO2Ph |
49.4 |
42.3 |
COMe |
48.3 |
41.0 |
CN |
48.0 |
39.8 |
SO2Me |
42.4 |
41.2 |
NO2 |
42.4 |
33.5 |
SO2CF3 |
|
31.4 |
a Chemical shift values upfield (from HCONH2 as external reference) in 1.7 M Me2CO-d6 solutions.
7. NMR of compounds containing NH2, NO2 and NO groups |
309 |
increasing -electron donation to the conjugated substituent from the deprotonation centre of the anionic forms. In the case of anilines in DMSO, hydrogen-bond solvation by DMSO of the NH groups increases the donation of -electrons to the conjugated CR substituent and this electron donation has been found to permit SSAR enhancement effects on the υ(15N) of the appropriate neutral aniline solutes39.
When υ(15N) values for the previously used series of 13 para-substituted anilines were measured in acetone42, a significantly weaker hydrogen-bond acceptor solvent than DMSO, a smaller shift dependence on para -electron-acceptor substituent solvation (SSAR) effects in acetone was observed (Table 10). This reduction42 was expressed by the (rather unsatisfactory) forms, 4 and 5.
−δ |
|
H |
OCMe2 |
−δ |
|
H |
OSMe2 |
|
+δ |
|
|
+δ |
|
||
X |
N |
|
X |
N |
|
||
|
|
|
|
||||
|
|
H |
OCMe2 |
|
|
H |
OSMe2 |
|
|
+δ |
|
|
|
+δ |
|
|
(4) |
|
|
|
(5) |
|
|
In the case of 5-substituted 2-nitroanilines (Table 11) it was observed42 that there are no significant SSAR effects for the meta-substituted compounds, in contrast to the relatively large SSAR N15 shifts observed39 for 4-substituted 2-nitroanilines in DMSO, which could be expressed by the resonance forms 6 843.
TABLE 11. 15N NMR chemical shifts of 4-substituted (I) and 5-substituted (II) nitroanilines39,42
|
|
X |
X |
NH2 |
NH2 |
|
NO2 |
NO2 |
( I ) |
|
( I I ) |
|
|
|
X |
υ15N (I)a |
υ15N (II)a |
OMe |
37.4 |
32.0 |
Me |
36.7 |
34.6 |
F |
35.8 |
31.4 |
Cl |
32.7 |
|
CF3 |
27.7 |
31.5 |
SCF3 |
27.0 |
|
H |
34.6 |
34.6 |
CO2Me |
25.4 |
|
CO2Et |
25.9 |
33.2 |
COMe |
24.7 |
33.0 |
CN |
23.4 |
|
SO2Me |
24.2 |
30.6 |
NO2 |
18.8 |
29.8 |
a Chemical shift values upfield (from HCONH2) as external reference in 1.7 M Me2SO-d6 solutions.
310 |
Edward W. Randall and Christiana A. Mitsopoulou |
|
||
|
|
H |
|
H+ |
|
|
|
|
|
X |
|
N |
− X |
N |
|
|
H |
|
H |
|
|
N |
N |
|
|
O |
O |
O |
O |
|
|
|
||
|
|
(6) |
(7) |
|
H+
X N
H
N
O O
(8)
B. Nitrobenzenes
1. Substituent effects on 15N chemical shifts
Substituent effects on 13C and 1H chemical shifts in the benzene ring of nitrobenzene derivatives are easily measurable and have been previously reported44,45. However, studies of substituent effects on nitro chemical shifts are more difficult because of the low inherent sensitivity of both the 15N and 17O nuclei46. Despite the growing literature on natural abundance 15N NMR spectroscopy46, until 1983 there had been relatively few reports47 49 pertaining to nitro groups. This was the result of several factors: the low natural abundance of 15N; the long spin relaxation times and small negative nuclear Overhauser effects. Optimization of pulse-flip angles and recycle times in the FT NMR experiment is required to overcome the first difficulty. The second difficulty, i.e. potential signal nulling arising from partial (negative) NOEs, can be overcome by gating the proton decoupler on only during data acquisition to remove long-range N H couplings without contributing to a buildup of NOE.
The nitrogen chemical shifts of aromatic nitro groups seem potentially to be a measure of the field-inductive effects of substituents.
Data for some nitrobenzene derivatives in DMSO and cyclohexane are collected50 in Table 12.
Because the difference in the shieldings between the two solvents for one nitrobenzene derivative is constant at almost 4.5 ppm, there is no evidence of any serious influence of substituents on the range and direction of solvent-induced variation in the shielding of the aromatic nitro group.
Some clear and important observations can be made from Table 12, if one excludes the 2-substituted nitrobenzenes from consideration, for which short-range effects can take place. If one compares only solutions in a given solvent, all of the substituents examined in position 3 as well as in position 4 seem invariably to induce an increase in the magnetic shielding of the nitrogen nucleus, with respect to that in nitrobenzene. Moreover, there is only a small difference, for a given substituent and solvent, between the meta and para effects on the shielding. The largest effects of this kind are exerted by an additional nitro

7. NMR of compounds containing NH2, NO2 and NO groups |
311 |
|||||
|
TABLE 12. Nitrogen NMR shieldings of a nitro group in |
|
||||
|
substituted nitrobenzenes50 (0.25 M solutions) |
|
|
|
||
|
|
|
Nitrogen shielding |
(ppm)a |
|
|
|
Substituent |
in cyclohexane |
in DMSO |
|
||
|
|
|
|
|
|
|
|
none |
12.62 |
8.32 |
|
|
|
|
2-Me |
7.34 |
3.26 |
|
|
|
|
3-Me |
12.27 |
8.23 |
|
|
|
|
4-Me |
12.63 |
8.46 |
|
|
|
|
2-OMe |
12.32 |
7.74 |
|
|
|
|
3-OMe |
12.71 |
9.17 |
|
|
|
|
4-OMe |
13.74 |
9.52 |
|
|
|
|
4-NMe2 |
|
|
10.11 |
|
|
|
2-F |
18.04 |
14.30 |
|
|
|
|
3-F |
15.87 |
11.46 |
|
|
|
|
4-F |
15.43 |
11.21 |
|
|
|
|
2-Cl |
13.63 |
9.21 |
|
|
|
|
3-Cl |
15.86 |
11.56 |
|
|
|
|
4-Cl |
15.30 |
10.74 |
|
|
|
|
2-Br |
12.34 |
7.23 |
|
|
|
|
3-Br |
15.95 |
11.76 |
|
|
|
|
4-Br |
15.23 |
10.75 |
|
|
|
|
2-I |
10.70 |
4.23 |
|
|
|
|
3-I |
15.93 |
11.35 |
|
|
|
|
4-I |
14.67 |
9.76 |
|
|
|
|
2-NO2 |
18.49 |
13.24 |
|
|
|
|
3-NO2 |
18.25 |
13.34 |
|
|
|
|
4-NO2 |
17.65 |
12.57 |
|
|
|
|
3,5-di-NO2 |
22.67 |
17.63 |
|
|
|
a Nitrogen shielding in ppm referenced to external neat nitromethane.
group, about C5 ppm, which is comparable to the range of solvent-induced variations in the shielding for a given substituent. Thus, solvent and substituent effects on the nitrogen shieldings in nitrobenzenes are comparable in magnitude, and an important conclusion follows that there is practically no sense in considering the latter for anything else than dilute solutions in a given solvent.
Comparison of substituent effects on the nitrogen shielding in substituted nitrobenzenes with substituent parameters44 showed that, whereas shifts for meta-substituted nitrobenzenes have a reasonable linear correlation with the m parameters, the para-substituted nitrobenzenes not only fail to give an analogous correlation with the parameter set, but also show evident discrepancies in the signs of substituent-induced changes in the nitrogen shielding with respect to the signs of the p parameters involved. Since the parameters considered are collective in the sense that they try to integrate all possible effects exerted by a given substituent in a given position in the aromatic ring, it can be concluded that the nitrogen shieldings of the nitro groups do not respond to the resonance or conjugative effects, and that they are sensitive primarily to the so-called field/inductive effects of substituents.
In the case of polyfluorinated38 nitrobenzenes, the signals of nitrogen are shifted ca 20 ppm upfield with respect to their hydrocarbon analogues (Table 9). The explanation is the same as in the case of the polyfluorinated anilines. The smaller upfield shift of 15N signals in 2,6-difluoronitrobenzene and N-sulphonyl-2,6-difluoroaniline compared to those of pentafluoro-substituted derivatives, especially in the case of 2,6-difluoronitrobenzene [ υ15N 14 ppm], can be interpreted in terms of a reduced conjugation of NO2 and NSO
312 |
Edward W. Randall and Christiana A. Mitsopoulou |
groups with the |
-systems of 2,6-difluorobenzene rings, but that effect is weaker in the |
case of pentafluorophenyl ones.
2. Substituent effects on 17O chemical shifts
Although Christ51 examined 17O shifts of some substituted nitrobenzenes 35 years ago, only at the beginning of the following decade did systematic studies of the substituent effects on 17O chemical shifts appear in the literature with the first reports52,53 of substituted anisoles, acetophenones and benzaldehides. The potential of this probe as a measure of substituent electronic effects was demonstrated.
Lipkowitz54 studied the 17O shifts of some meta- and para-substituted nitrobenzenes. However, the high concentrations and small range of substituents investigated make these data of limited use.
Some time later the feasibility of obtaining 17O spectra for natural abundance samples at relatively low concentration was demonstrated55,56. This was an important step because intermolecular interactions can markedly affect chemical shifts and, if only electronic substituent effects are to examined, then all extraneous influences must be minimized.
The study55 of the 17O spectra of nine para-substituted nitrobenzenes, whose electrondonating or -attracting groups cover a 3-fold larger range of values than those which had been studied by Lipkowitz54, revealed that the 17O shieldings are strongly influenced by the nature of the para substituent and in a manner completely consistent with the existing valence bond theory.
Substituent effects on the 17O chemical shifts in meta- and para-substituted nitrobenzenes are presented in Table 13, showing that these shifts are quite sensitive to the nature of the substituent, and range over nearly 40 ppm. Examination of Table 13 shows that the direction of substituent effects is different for 15N and 17O shifts, with electron-withdrawing substituents (such as NO2, CN or CF3) causing upfield 15N and
TABLE 13. 17O shieldinga data for some meta- and para-substituted nitrobenzenes56 (3- or 4-X-C6H4NO2)
|
|
|
υ17O (ppm) |
X |
|
para |
meta |
|
|
|
|
NEt2 |
33.4 |
|
|
NH2 |
25.6 |
0.8 |
|
OMe |
9.8 |
|
|
OH |
6.7 |
|
|
F |
1.3 |
2.9 |
|
Cl |
0.6 |
|
|
Br |
|
|
4.0 |
Me |
3.3b |
0.4 |
|
H |
0.0 |
0.0 |
|
CHO |
8.1 |
1.9 |
|
CF3 |
7.7 |
2.7 |
|
CN |
7.5 |
3.5 |
|
NO2 |
11.5 |
5.0 |
|
COMe |
3.3 |
|
|
COOMe |
7.2 |
|
|
a Positive shifts are downfield relative to H2O. Error š0.1 ppm. bNitrobenzene has an 17O chemical shift ca 569 ppm downfield from H2O.
7. NMR of compounds containing NH2, NO2 and NO groups |
313 |
||||||||||
δ+ |
|
δ+ |
δ+ |
δ+ |
|
||||||
|
|
|
|
− |
− |
||||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
+ |
|
|
|
|
N |
|
|
|
N |
|
N |
|
|
||
δ+ |
|
δ− |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
δ− |
|
|
δ− |
|
|
+ |
|
||
|
δ+ |
|
δ+ |
|
|
δ− |
|||
|
|
|
|
|
|
||||
|
|
|
|
|
|
||||
X |
δ− |
X |
δ− |
X |
δ+ |
||||
(a) |
(b) |
(c) |
|||||||
|
|
|
|||||||
FIGURE 2. -Polarization |
effects: (a) separate localized polarization of the |
side-chain and ring |
|||||||
systems: (b) extended polarization of the ring and the side-chain conjugated |
system: (c) possible |
||||||||
representation of extended polarization in terms of field-induced resonance. The dipolar substituent X stabilizes particular resonance forms, thus bringing about enhanced electron density changes at conjugated sites such as the terminal oxygen atoms. Note that the substituent shown has an electrondonating polar effect (e.g. a methyl group). Electron-withdrawing substituents, which have a dipole
opposite to that shown, would destabilize this resonance form. Reprinted with |
permission from |
P. Balakrishnan and D. W. Boykin, J. Org. Chem., 50, 3663 (1985). Copyright |
(1985) American |
Chemical Society |
|
downfield 17O shifts56. This multinuclear approach to the study of substituents effects proves the generality of the -polarization concept by showing that distant substituents can readily polarize the electrons of an NO bond (see Figure 2).
The
Table 14.
It can be seen that there is a deshielding of oxygen for each case, compared with the hydrocarbon analogue, which confirms a reduced conjugation of the NO2 (and NSO) group with the -systems of polyfluorobenzene rings38.
The values for the 17O chemical shifts for ˇ-nitrostyrenes (9) in acetonitrile at 70 ° C are given in Table 14; the chemical shifts of these compounds are in the region reported for nitrobenzenes57. Electron-attracting substituents cause deshielding of the nitro signal whereas electron-donating groups produced shielding58.
NO2
X
(9)
314 |
Edward W. Randall and Christiana A. Mitsopoulou |
TABLE 14. 17O chemical shift of some nitro compounds containing aromatic derivatives (ppm vs H2O)
Compound |
υ17O |
Compound |
υ17O |
17O Chemical shifts (−NO2) ppm nitrostyrenes
C6F5NO2 |
627 š 4 |
PyFNO2 |
632 š 3 |
C6F5NSO |
437 š 3 |
4-CF3C6F4NSO |
441 š 6 |
2-NH2C6H4NO2 |
558 |
4-NMe2C6H4CHDCHNO2 |
562.4 |
3-NH2C6H4NO2 |
575 |
4-OHC6H4CHDCHNO2 |
572.0 |
4-NH2C6H4NO2 |
585 |
4-OMeC6H4CHDCHNO2 |
573.0 |
2-NO2C6H4NO2 |
608 |
4-MeC6H4CHDCHNO2 |
577.1 |
3-NO2C6H4NO2 |
570 |
4-FC6H4CHDCHNO2 |
578.4 |
4-NO2C6H4NO2 |
585 |
C6H5CHDCHNO2 |
578.8 |
2-ClC6H4NO2 |
610 |
4-ClC6H4CHDCHNO2 |
579.5 |
3-ClC6H4NO2 |
567 |
4-BrC6H4CHDCHNO2 |
579.6 |
C6F5NO |
699 š 2 |
4-CF3C6H4CHDCHNO2 |
582.1 |
4-NEtC6F4NO2 |
644 š 4 |
4-CNC6H4CHDCHNO2 |
583.5 |
4-MeOC6F4NO2 |
633 š 4 |
4-NO2C6H4CHDCHNO2 |
586.2 |
4-HC6F4NO2 |
631 š 3 |
|
|
p-NO2 
585
p-CN 
 p-CF3
p-CF3
580 p-Cl
p-F |
H |
|
|
p-Me |
|
575
 p-MeO
p-MeO
p-OH
570 |
565 |
570 |
575 |
580 |
560 |
17O Chemical shifts (−NO2) nitrobenzenes (ppm)
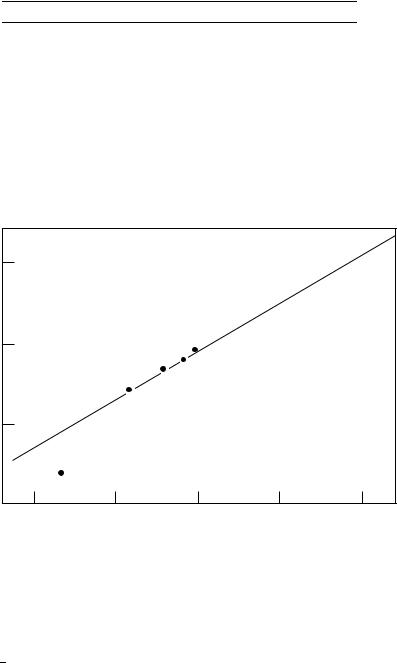
FIGURE 3. Plot of 17O chemical shift (NO2) nitrobenzenes vs 17O chemical shift (NO2) ˇ-nitrostyrenes in CH3CN. Reproduced from Reference 58 by permission of Elsevier Science B.V.
The range of SCS values (p-CH3O to p-NO2) for nitrostyrenes is about 13 ppm. The correlation of the 17O chemical shift data for nitrostyrenes with that for nitrobenzenes57 gives a slope of 0.58 (Figure 3), which indicates that a comparable reduction in substituent effects results when the nitro function is separated from a p-substituted phenyl group by a carbon carbon double bond.
