

Supplement A3: The Chemistry of Double-Bonded Functional Groups. Edited by Saul Patai Copyright 1997 John Wiley & Sons, Ltd.
ISBN: 0-471-95956-1
CHAPTER 26
N-Oxidative transformations of C=N groups as means of toxification and detoxification
PETER HLAVICA and MICHAEL LEHNERER
Walther-Straub-Institut fur¨ Pharmakologie and Toxikologie, Ludwig-Maximilians- Universitat¨ Munchen,¨ Nussbaumstrasse 26, D-80336 Munchen,¨ Germany
Fax: 49-89-5145-2224; e-mail: Michael.Lehnerer@lrz.uni-muenchen.de
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1626 |
|
II. CHEMICAL ASPECTS OF THE N-OXYGENATION OF THE CDN |
|
|
FUNCTIONALITY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1626 |
|
A. Types of CDN Functionalities Prone to N-Oxygenation and Chemical |
|
|
Characteristics of the N-Oxygenated Products . . . . . . . . . . . . . . . . |
1626 |
|
B. Methods for the Detection of Oximes, Nitrones and N-Oxides . . . . . |
1628 |
|
1. |
Paper and thin-layer chromatographic (TLC) methods . . . . . . . . |
1628 |
2. |
Gas liquid chromatographic (GLC) methods . . . . . . . . . . . . . . |
1629 |
3. |
High-performance liquid chromatographic (HPLC) methods . . . . |
1629 |
4. |
Nonchromatographic methods . . . . . . . . . . . . . . . . . . . . . . . . |
1630 |
III.OCCURRENCE OF N-OXYGENATED CDN FUNCTIONALITIES IN NATURAL AND SYNTHETIC COMPOUNDS AND
BIOLOGICAL ACTIVITY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1630 |
A. Occurrence in Microorganisms and Plants . . . . . . . . . . . . . . . . . . |
1630 |
B. Occurrence in Synthetic Compounds . . . . . . . . . . . . . . . . . . . . . |
1632 |
IV. METABOLIC IN VIVO AND IN VITRO FORMATION OF |
|
N-OXYGENATED CDN FUNCTIONALITIES . . . . . . . . . . . . . . . . |
1633 |
A. Formation of Oximes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1633 |
B. Formation of Nitrones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1635 |
C. Formation of N-Oxides . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1636 |
V. ENZYMOLOGY OF THE FORMATION OF N-OXYGENATED CDN |
|
FUNCTIONALITIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1639 |
A. Enzymology of Oxime Formation . . . . . . . . . . . . . . . . . . . . . . . |
1639 |
B. Enzymology of Nitrone Formation . . . . . . . . . . . . . . . . . . . . . . . |
1643 |
C. Enzymology of N-Oxide Formation . . . . . . . . . . . . . . . . . . . . . . |
1647 |
1625

1626 |
Peter Hlavica and Michael Lehnerer |
|
VI. FURTHER TRANSFORMATIONS OF N-OXYGENATED CDN |
|
|
FUNCTIONALITIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1650 |
|
A. Enzymatic Processes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1650 |
|
1. |
Reductions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1650 |
2. |
Oxidations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1651 |
3. |
Deaminations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1652 |
4. |
Isomerization and rearrangement reactions . . . . . . . . . . . . . . . . |
1653 |
5. |
Conjugations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1653 |
B. Nonenzymatic Processes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1655 |
|
1. |
Nucleophilic reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1655 |
2. |
Photochemical reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1656 |
3. |
Radical reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1657 |
VII. CONCLUSIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1658 |
|
VIII. REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1659 |
|
|
|
|
I. INTRODUCTION
The presence of a CDN functionality in xenobiotics, including drugs, is not uncommon as this constituent can occur in aliphatic, alicyclic, aromatic and heteroaromatic structures. Parli and coworkers were the first to report on the biological N-oxygenation of 2,4,6-trimethylacetophenone imine by rat and rabbit liver microsomal fractions to yield a stable oxime1. Similarly, amidines, a class of strongly basic imines, have been shown to undergo N-oxidative transformation to the corresponding amidoximes2. Subsequent studies with diarylimines indicated that microsomal oxygenation may lead to the formation of diarylnitrones3. Administration of bromazepam, a cyclic imine, to dogs has been found to result in nitrone production as a minor urinary pathway4. Interest in the role of oximes and nitrones in drug metabolism was enhanced upon the discovery of the occurrence of these N-oxy compounds as intermediates in the hepatic turnover of amphetamines5,6 eliciting stimulant actions in the central nervous system. Pyridine, bearing a CDN group as part of a heteroaromatic nucleus, is an industrial chemical used as a solvent and as an intermediate in the synthesis of pharmaceuticals, paints and insecticides and has been detected to be metabolically converted to the N-oxide by various animal species7, as is also the case with some aromatic diazines8,9.
Particular accounts on the biochemistry and pharmacology of N-oxygenation of endogenous and exogenous compounds containing CDN group(s) have not been previously given. The present chapter thus undertakes to collate available data on this subject. Moreover, this compilation of information is hoped to focus attention on this specific area of nitrogen oxidation and promote research in this field.
II. CHEMICAL ASPECTS OF THE N-OXYGENATION OF THE C=N
FUNCTIONALITY
A. Types of C=N Functionalities Prone to N-Oxygenation and Chemical
Characteristics of the N-Oxygenated Products
Table 1 summarizes the prototypes of CDN functionalities susceptible to N-oxygenation in biological systems. Formation of imines from amines means a transition of the nitrogen electrons from sp3 to sp2 hybridization with a concomitant lowering of the basicity10, the oxidation state of the constituent nitrogen in the imino group11 being defined as3. Basically, there exists an imine enamine tautomerism, the equilibrium being largely shifted toward the imine structure (equation 1). Comprehensive studies on the metabolism

26. N-Oxidative transformations of CDN groups |
1627 |
of imines have been frequently hampered by the fact that simple imines are readily hydrolyzed to ketones in the presence of moisture or dilute acids12. However, using a series of sterically hindered imines resisting hydrolysis, N-oxidative turnover could be successfully assessed1,13. Similarly, amidines and guanidines, representing strong bases containing double-bonded nitrogen atoms, are stabilized by resonance upon protonation to permit metabolic investigation2,14.
R2 |
|
|
R2 |
R1CH2 C |
|
R1CH C |
(1) |
|
|||
NH |
|
|
NH2 |
Oxidation of imine, amidine and guanidine nitrogens yields oximes, oxidative attack at the nitrogen centers in alkyl-, arylor cyclic imines affords nitrones. The oxidation state of nitrogen in these functions11 is defined as 1. Both oximes and nitrones exist as a mixture of two geometric isomers, Z and E, previously termed syn and anti, respectively
TABLE 1. Prototypes of CDN functionalities prone to metabolic N-oxygenation
Type of |
N-Oxygenated |
Model compounds |
Reference |
compound |
product |
studied |
|
RCHDNH |
|
RCHDNOH |
|
|
(Aldimine) |
|
(Aldoxime) |
|
|
RC(R1)DNH |
RC(R1)DNOH |
|||
(Ketimine) |
|
(Ketoxime) |
|
|
RC(NH2)DNH |
RC(NH2)DNOH |
|||
(Amidine) |
|
(Amidoxime) |
|
|
RNHC(NH2)DNH |
RNHC(NH2)DNOH |
|||
(Guanidine) |
(Guanidoxime) |
|||
|
|
O |
|
|
RCHDN-R |
1 |
j |
R |
1 |
|
RCHD N |
|
||
|
|
C |
|
|
(N-Substituted |
(Nitrone) |
|
|
|
imine) |
|
|
|
|
C |
C |
N |
+ |
N |
|
|
O− |
(Cyclic imine) |
(Cyclic nitrone) |
N |
N |
|
|
|
O |
(Heteroaromatic |
(N-oxide) |
amine) |
|
Butyraldimine |
179 |
|
|
Acetophenone |
1,13,85 |
||
imines |
|
|
|
Benzamidines |
2,47 |
|
50,86 |
|
|||
Pentamidine |
51 |
|
|
Debrisoquine |
52 |
|
|
Diarylimines |
3,31 |
|
|
Bromazepam |
4 |
|
|
Methaqualone |
43,89 |
|
|
Quinolines |
7,18,28,32,41,62,96 |
Pyridines |
15,28,104 |
Pyrimidines |
8,16,105,106 |
Purines |
64,65,107 |
Pyridazines |
9,108 |
Pyrazines |
9 |
Triazines |
109 |

1628 |
Peter Hlavica and Michael Lehnerer |
(equations 2 and 3). Under normal conditions, some nitrones are reasonably stable, while others are susceptible to nucleophilic attack and hydrolyze to give primary hydroxylamines and electrophilic aldehydes. Hydrolysis is acid catalyzed, but also occurs at a perceptible rate in neutral solutions at ambient temperature. Nitrones with an aryl substituent at the ˛-carbon are more resistant to hydrolysis owing to the presence of a double bond in conjugation with an aromatic ring. N-Alkylnitrones generally hydrolyze readily; however, stability increases with the size of the N-alkyl group.
R1 OH R1
C |
N |
C |
N |
(2) |
|
|
|||||
R2 |
|
R2 |
|
OH |
|
R1 |
O− |
R1 |
+ |
R3 |
|
|
+ |
|
(3) |
||
|
C |
N |
|||
C |
N |
||||
|
|
|
|||
R2 |
R3 |
R2 |
|
O− |
The class of heteroaromatic amines known to undergo biological N-oxygenation includes pyridine (pKa D 5.2), quinoline (pKa D 4.85), isoquinoline (pKa D 5.14), pyridazine (pKa D 2.33), pyrimidine (pKa D 1.3) and pyrazine (pKa D 0.6) structures7,9,15,16. As is evident, basicity of these compounds is highly divergent. N- Oxygenation of the heterocyclic ring systems produces the corresponding N-oxides, a reaction requiring the participation of the lone pair of electrons on the vulnerable nitrogen in the bonding orbital linking the nitrogen and oxygen atom; this process is associated with a change in the oxidation state of the nitrogen from 3 to 1. N-Oxide formation results in a considerable decrease in the pKa of the amines15. Because of resonance stabilization, heteroaromatic N-oxides are chemically more stable and less sensitive to heat as compared with other classes of N-oxides16,17. Since no completely uncharged Lewis structure may be written for N-oxides, both the oxygen and nitrogen have octet configuration and bear ( ) and (C) formal charges, respectively. When metabolically formed, the polarity of heteroaromatic N-oxides thus strongly favors their urinary excretion18. N-Oxide formation, therefore, has been considered a route of detoxication of foreign compounds11.
B. Methods for the Detection of Oximes, Nitrones and N-Oxides
The detection of products derived from the N-oxygenation of CDN functionalities presents many problems, which illustrate difficulties that are associated with the isolation, identification and quantification of small amounts of water-soluble metabolites. Spectrophotometric methods19 as well as differential pulse polarographic techniques20 previously used to determine oximes, nitrones and N-oxides frequently lack sensitivity and/or specificity. Improved analytical methods for the quantification of these N-oxy compounds include chromatographic techniques taking into account the chemical peculiarities of the individual N-oxygenated CDN functionalities. These procedures usually require the chemical synthesis of authentic material for comparison with data obtained with the isolated metabolites, and also for the construction of calibration curves.
1. Paper and thin-layer chromatographic (TLC) methods
TLC has been found to be useful in the separation of 4-hydroxyphenylacetaldoxime generated from L-tyrosine in E.coli cell cultures21. This method has been also employed to
26. N-Oxidative transformations of CDN groups |
1629 |
detect small amounts of isomeric acetaldoximes formed from SKF 40652A, a secondary phenylethylamine22; visualization of the N-oxygenated material was achieved by treatment after development of the chromatograms with specific chromogenic reagents or inspection under UV light. Using TLC as an analytical means, oximes were recognized to be metabolites of promazine and chlorpromazine23. This technique also permitted detection of oximes isolated from incubation media containing a series of substituted acetophenone imines13. Moreover, TLC served to analyze amidoximes produced from ring-substituted benzamidines2. Similarly, this method was apt to identify small amounts of nitrones formed from N-substituted amphetamines24,25 or 4-substituted N-benzylanilines26.
Multiple TLC systems have been developed taking advantage of a variety of polar solvents for the separation and identification of N-oxides derived from heteroaromatic amines, such as ring-substituted pyridines, quinolines, isoquinolines and quinoxalines18,27 29. However, these studies were unable to describe a chromogenic reagent generally applicable to the detection of the N-oxide function in the heterocycles examined. Ascending paper chromatography30 along with TLC was also used to identify N-oxide metabolites originating from organic compounds containing a pyridyl nucleus31,32. Finally, TLC has proved to be useful in the separation of N-oxides arising from metabolic transformation of pyridazines, pyrimidines and pyrazines8,9. Identification of trace amounts of metabolites was often aided by the application of radiolabeled compounds21,32 permitting autoradiography of the TLC plates.
2. Gas liquid chromatographic (GLC) methods
GLC techniques, frequently combined with mass-spectral analysis to confirm the structures of the separated metabolites, have been applied to the detection of aldoximes and ketoximes generated from phenothiazines23, acetophenone imines1,13, phenylethylamines5,22,33 37 and the phenoxyaminopropane compound mexiletine38. Quantification of these products by direct GLC has not always been possible because of their thermolability. This made necessary the development of methods permitting conversion of the substances to heat-stable derivatives, as was achieved by treatment with trimethylsilylating or trifluoroacetylating agents5,22,23,33 35,37.
Direct GLC has also been used to separate synthetic or metabolically formed nitrones derived from N-substituted phenylethylamines6,24,25,36,39. As certain ring-substituted pyridines are thermostable and sufficiently volatile, Gorrod and Damani succeeded to establish procedures for direct GLC analysis of some pyridine N-oxides18,40,41. It has to be noted that N-oxides derived from the bispyridyl compound metyrapone undergo catalytic deoxygenation on the glass column during GLC separation to yield a single peak corresponding to that of metyrapone42. Similar observations were made with methaqualone N-oxide, a quinazolinone derivative43. Instable heteroaromatic N-oxides thus were quantitated after reduction by TiCl3 to their parent amines43,44.
3. High-performance liquid chromatographic (HPLC) methods
HPLC techniques have proved to be a major advance in direct analysis in a nondestructive manner of compounds bearing an N-oxygenated CDN functionality. Reverse-phase HPLC has been used for the separation of aldoximes45, ketoximes46, amidoximes14,47 52 and nitrones3,26,53 55. This method also served to identify N- oxides derived from pyridines7,29,31,56 63, pyrimidines8,9,16, pyrazines9, pyridazines9 and purines64,65; the latter class of heteroaromatic N-oxides was analyzed on cationic exchange columns.

1630 |
Peter Hlavica and Michael Lehnerer |
4. Nonchromatographic methods
In many instances, oximes13,22,23, nitrones24,25,36 and N-oxides9,18,29 isolated by the chromatographic procedures decribed above have been subjected to mass-spectral analysis to verify their chemical nature. The majority of oximes derived from phenylethylamines produced spectra with fairly abundant molecular ions. Oximes with a benzyl group gave a base peak at m/z 91 for this portion of the molecule. Diagnostic ion peaks were generally observed at M 17 C and M 33 C. Nitrones produced from N-substituted amphetamines displayed weak molecular ion peaks and a diagnostic peak corresponding to M R C , where R is the mass of the N-alkyl group minus 14. N-Oxides arising from oxygenation of pyridines displayed a molecular ion peak in each case and a diagnostic (M 16 C ion peak corresponding to the loss of an oxygen atom. An M 17 C peak was shown to be due to the loss of oxygen followed by the loss of H. Similarly, a series of quinoline and isoquinoline N-oxides gave relatively weak M 16 C ion peaks. The subject has been reviewed by Cowan66.
Alternatively, oximes13,23, nitrones3,24,39,55 and N-oxides30 have been identified by proton nuclear magnetic resonance (1H-NMR) spectroscopy. In addition, 15N NMR techniques have been made available for the structural analysis of amidoximes and guanidoximes50,52. Owing to the spin of 1/2 of the 15N nucleus, this nitrogen isotope is suitable for high-resolution experiments although its natural abundance of 0.37% causes but a very small magnetogyric ratio. This difficulty can be met by application of the pulse Fourier transform method. Despite this, there is a relative large requirement for material naturally abundant in 15N to obtain spectra of good quality. Spectroscopic data obtained in this way exhibited chemical shifts for imine-type nitrogens in the middle-deshielded region of 380 150 ppm and coupling constants in the range of 60 80 Hz. For details, reference should be made to a recent account by Clement and Kampchen¨67.
III. OCCURRENCE OF N-OXYGENATED C=N FUNCTIONALITIES IN NATURAL
AND SYNTHETIC COMPOUNDS AND BIOLOGICAL ACTIVITY
A. Occurrence in Microorganisms and Plants
Iodinine (1), the pigment of Chromobacterium iodinum, was the first discovery of a heteroaromatic N-oxide68. The compound exhibits close structural relationship to myxin
(2), which possesses antibacterial activity69. Other naturally occurring di-N-oxides include the antibiotics pullcheriminic acid (3) and mycelianamide (4) isolated from various bacterial strains70. Further, aspargillic acid (5), purified from Aspergillus flavus, and emimycin
(6) represent pyrazine N-oxides characterized by antibacterial potency15,71. Mycobactin P (7) has been obtained from Mycobacterium phlei and is a potent growth factor for
O |
OH |
O |
OH |
O |
|
|
|
|
|
||
N |
|
N |
HO |
N |
But |
N |
|
N |
But |
N |
OH |
OH |
|
OMe |
|
O |
|
O |
|
O |
|
|
|
(1) |
|
(2) |
|
(3) |
|

|
|
|
26. N-Oxidative transformations of CDN groups |
1631 |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
N |
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
Me2 C |
|
|
CH(CH2 )2 C(Me) |
|
CHCH2 |
|
|
O |
|
Ph |
|
CH |
|
|
N |
|
|
|
OH |
|||
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4) |
|
|
|
|
|
|
|
|
O |
|
|
|
|
||
|
|
|
N |
|
But |
|
|
N |
OH |
|
|
|
|
|
|
|
|
|||||
Bui |
|
N |
|
OH |
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
O |
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
(5) |
|
|
|
|
(6) |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(CH2 )4 |
N C(OH)CH |
|
CH(CH2 )14 Me |
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
HO |
|
|
O |
|
|
|
|
|
|
|
|
||
OH |
|
|
O |
O |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
H |
|
|
|
|
N |
|
|
|
|
|
|
|
|
||||||||
|
|
|
CNHCHCOCHCHCNH |
|
|
|
|
|
|
OH |
O |
|||||||||||
|
|
N |
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
Et Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
O |
|
|
|
|
|
|
|
|
HC |
|
|
N |
|
CH2 COOH |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
(7) |
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(8) |
||||||
Mycobacterium johnei72. Hadacidin (8) is produced by several Penicillia; its antitumor activity has been related to its antagonism to aspartic acid in adenylate synthesis73.
N-Oxygenated CDN functionalities also occur in plants. Thus, harman N-oxide (harmanine, 9) is the main alkaloid in all parts of Calligonium minimum74. The trunk bark of Aspidosperma nigricans contains the unstable olivacine N-oxide (10)74. Orellanine (11) has been isolated from the toadstool Cortinarius orellanus75. The substance is heat-stable and exerts considerable nephrotoxicity.
O
N
|
|
N |
|
N |
|
N |
|
|
|
O |
|
H |
Me |
H |
Me |
|
(9) |
|
(10) |
1632 |
Peter Hlavica and Michael Lehnerer |
|||
|
HO |
|
OH |
O |
|
|
|
||
|
|
|
|
N |
|
|
N |
|
|
|
|
O |
HO |
OH |
|
|
|
|
|
(11)
B. Occurrence in Synthetic Compounds
Although a broad spectrum of pharmaceuticals containing an N-oxygenated CDN functionality has been tested for pharmacological activity, only a few types of compounds proved useful in modern therapy.
The ketoxime derivative fluvoxamine (12) is a newer antidepressant thought to potentiate the action of 5-hydroxytryptamine76. Oxacillin (13), cefuroxime (14) as well as the monobactam aztreonam (15) represent potent antibacterial agents of the beta-lactam type77. The aldoxime pralidoxime (16) and a number of bis-quarternary oximes, such as obidoxime (17), can be used as reactivators of the phosphorylated esteratic site of acetylcholinesterase that occurs in the presence of organophosphate inhibitors78,79.
|
|
|
|
|
|
|
|
Me |
F3 C |
C |
Pen |
Ph |
|
|
CONH |
|
S |
|
|
|
|
|
|
Me |
||
|
N |
|
|
N |
|
|
N |
COOH |
|
O(CH2 )2 NH2 |
|
O |
Me O |
||||
|
|
|
|
|
||||
|
(12) |
|
|
|
|
(13) |
|
|
|
|
C |
CONH |
|
S |
|
|
|
|
|
|
|
|
|
|
||
|
|
O |
|
|
|
|
|
|
|
|
N |
OMe O |
N |
|
CH2 OCONH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COOH |
|
|
|
|
|
|
(14) |
|
|
|
|
|
N |
|
|
|
|
|
|
CH |
NOH CH NOH |
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2 N |
C CONH |
|
|
|
|
|
|
|
S |
O |
N |
|
|
|
|
|
|
|
N |
|
CH |
NOH |
|
|
||
|
O |
Me |
SO3 H +N |
+N |
+N |
|||
|
|
|
||||||
|
|
C |
|
Me |
|
|
CH2 |
O CH2 |
|
|
|
|
|
|
|
|
|
|
Me |
COOH |
|
|
|
|
|
|
|
(15) |
|
(16) |
|
|
(17) |
||
Similarly, the category of N-oxides comprises compounds of considerable pharmacological interest. Chlordiazepoxide (18) possesses potent antidepressant, sedative, anticonvulsant and muscle relaxant properties80. Minoxidil (19) is an antihypertensive of the vasodilatator type. Ciclopirox (20) shows antifungal activity81.
|
26. N-Oxidative transformations of CDN groups |
|
1633 |
||||
|
|
NHMe |
O |
|
|
O |
|
|
N |
|
|
|
|
|
|
|
|
N |
|
|
N |
|
|
|
|
H2 N |
NH2 |
c-Hex |
OH |
||
Cl |
|
N |
N |
|
|
|
|
|
Ph |
O |
Pip |
|
|
Me |
|
|
|
|
|
|
|||
|
|
|
|
|
|
||
|
(18) |
|
(19) |
|
|
(20) |
|
Within the class of N-oxy compounds designed for experimental use, the tremorogenic action of Z-thiophene-2-aldoxime (21) has been extensively studied82. Tremor is preceded by hyperpnoea and increased locomotor activity. Animals exhibit concurrent behavioral depression and ptosis. Nitroquinoline N-oxide (22) is a potent carcinogen, in which the N O function appears to be essential to carcinogenic activity; conversion to the proximate carcinogen requires reduction of the nitro group to yield 4-hydroxylaminoquinoline N-
oxide83. Similarly, some purine N-oxides have been demonstrated to induce malignant tumors84.
NO2
S
CH NOH
N
O
(21) |
(22) |
IV. METABOLIC IN VIVO AND IN VITRO FORMATION OF N-OXYGENATED
C=N FUNCTIONALITIES
A. Formation of Oximes
Conversion of acetophenone imines to the corresponding ketoximes (23) has been studied both in vivo and in vitro. Thus, small amounts of 2,4,6-trimethylacetophenone oxime were found to be excreted in the urine of male rats dosed with the parent imine1. The oxime was also detected to arise from N-oxygenation of 2,4,6-trimethylacetophenone imine in aerobic incubation mixtures containing rat or rabbit liver microsomal fraction fortified with NADPH1. These observations were extended to other chemically stable substituted acetophenone imines, and it was shown that hepatic microsomal preparations from various mammalian species catalyzed biotransformation to differing extents, rates of isomeric oxime formation increasing in the order ferret < guinea-pig < mouse < rat < hamster < rabbit13,85.
Incubation of benzamidine and its ring-substituted congeners with 9000 g supernatant fraction from rabbit liver2,47,49 yielded the corresponding benzamidoximes (24a). The antiprotozoal drug pentamidine, which can be regarded a diamidine, has been found to undergo N-oxidative transformation to the monoamidoxime (25) in human and rabbit liver

1634 |
Peter Hlavica and Michael Lehnerer |
|
R |
C NOH |
R1 |
Me
C NOH
R2 NH
(23) |
(24) |
(a)R1 = H, Me, CN, Cl; R2 = H
(b)R1 = H; R2 = Me, t-Bu, Ph
HN |
|
|
NOH |
|
|
|
|
C |
O(CH2 )5O |
|
|
H2 N |
|
|
NH2 |
|
|
|
|
|
(25) |
|
|
|
|
|
Cl |
N |
NOH |
|
NOH |
|
|
|
NH |
|
NH2 |
H2 N |
NH2 |
|
|
N |
|
|
|
|
Ph |
(26) |
|
|
(27) |
microsomal suspensions51. Similarly, amidoximes (24b) could be isolated from incubates of N-monosubstituted benzamidines with hepatic preparations from the rabbit50,86. The latter system was about 3 times as effective as rat liver microsomes in N-oxygenating debrisoquine52, a guanidine derivative, to give the corresponding guanidoxime (26). NADPH-dependent enzyme sources from rat and rabbit liver also served to convert the aminoguanidine group of 2-amino-5-chloro-benzophenone amidinohydrazone14, an antiarrhythmic agent, to the oxime (27).
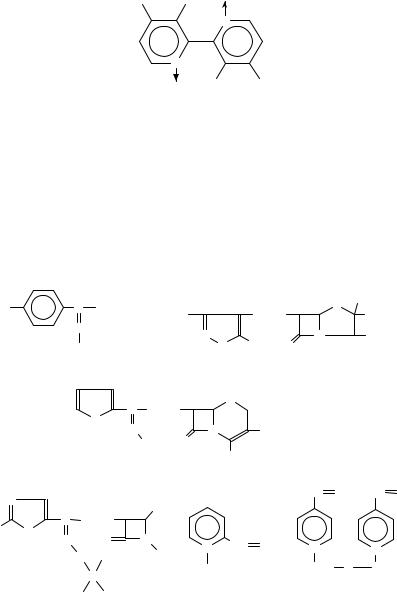
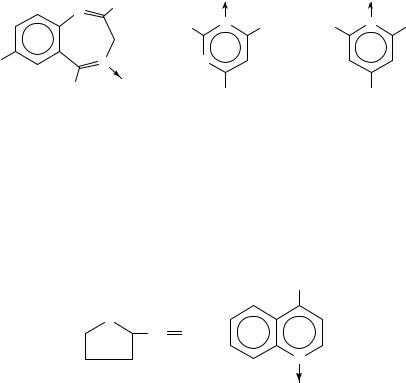
Moreover, oximes have been recognized to arise from oxidative attack at the nitrogen center of primary arylalkylamines. Thus, phenylacetone oxime (28) was detected in reaction media containing amphetamine and rabbit liver preparations5,33,34. Analogously, (2,4,6-trimethylphenyl)ethylamine37 gave the corresponding acetophenone oxime (23). Using washed hepatic microsomes from rabbits, hamsters and guinea-pigs, Gorrod and Raman succeeded to demonstrate the formation of a mixture of isomeric ketoximes (29) from o-methylbenzhydrylamine87. Extracts of urine specimens obtained from rats dosed with dezocine, a bridged aminotetralin derivative, have been shown to contain small amounts of a ketoxime (30) metabolite88.
Incubation of certain secondary22 and tertiary23 alkylamines with rabbit liver preparations has been reported to yield aldoximes upon N-oxygenation.
