
20. Epoxidation of CDX double bonds |
1243 |
Of peracids, MCPBA and (C)-MPCA are used most often. Oxidation is usually carried out in aprotic solvents, mostly in CH2Cl2, CHCl3, or with phase-transfer catalysis. Perfluorinated oxaziridines are prepared in acetonitrile212,213. The acid side-product, m-chlorobenzoic acid, is insoluble in the solvent and the desired oxaziridine may be prepared in good yield. MCPBA, however, is expensive, and large-scale oxidations are sometimes contaminated with bis(m-chlorobenzoyl) peroxide, which complicates product purification206.
Oxone is an inexpensive and stable oxidizing reagent that is commercially available. Replacement of MCPBA by buffered oxone in toluene results in increased yields, a significant reduction of the reaction time and easier purification of the oxaziridine206. In addition, other oxidizing agents were also employed for the epoxidation of imines214 218.
Epoxidation of both aldimines and ketimines is possible. Most oxaziridines formed are stable compounds, especially aldimines containing aromatic substituents, and 2-sulfonyl- and 2-sulfamyl oxaziridines5. Generally, N-sulfonyloxaziridines are isolated as stable crystalline solids. Certain compounds are widely used in synthetic organic chemistry as oxygen-transfer reagents (15 17).
|
|
Me |
|
Me |
|
R = Ph, Bu |
|
|
Me |
|
Me |
|
|
O |
|
R |
|
|
X |
X = Cl, OMe |
|
|
|
|
|
|
|
N |
C |
|
|
|
X |
|
|
|
|
|
|
|
|
PhSO2 |
|
H |
N: |
Me |
|
|
R = Ph, C6 H4 NO2 |
|
N: |
|
|||
SO2 |
O |
O |
|
|||
|
|
|
SO2 R |
|||
|
|
|
|
|
||
|
|
|
|
|
|
|
(15) |
|
(16) |
|
(17) |
|
|
Biphasic buffered oxidation of sulfonylimines usually suffice for stereoselective synthesis of trans-sulfonyl substituted oxaziridines in excellent yields (equation 41)203.
MCPBA
PhSO2 N CHPh BnEt3 N+Cl−/NaHCO3
O Ph
N C |
(41) |
PhSO2 H
Chiral 2-sulfonyl- and 2-sulfamyloxaziridines were also prepared in different ways4,5. Two examples are given in equations 42 and 43207.
Me |
Me |
|
|
Me |
Me |
|
|
oxone |
|
(42) |
|
N: |
|
N: |
|
SO2 |
SO2 |
O |
|
(−) |
(+) |
||
|
1244 |
Mihaly´ Bartok´ and Gyula Schneider |
|
|
|
Me |
Me |
Me |
|
Me |
|
|
|
|
oxone |
(43) |
|
|
|
|
|
:N |
:N |
|
|
SO2 |
O SO2 |
|
|
(+) |
(−) |
|
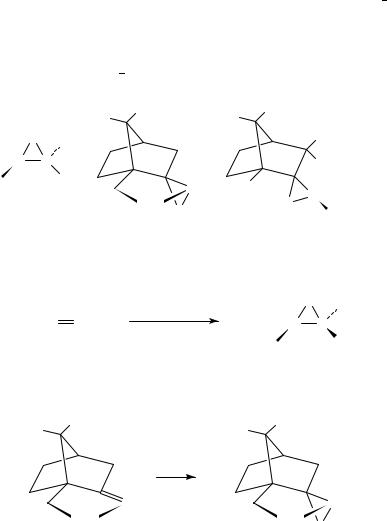
Both isomeric forms of (C)- and ( )-(camphorylsulfonyl)oxaziridines are available by oxidation of the corresponding sulfonimines with buffered potassium peroxymonosulfate (oxone). Since oxidation can only take place from the endo-face of the CDN double bond due to steric blocking of the exo-face, a single oxaziridine isomer is obtained. The enantiomerically pure sulfonimines can be prepared in three steps in better than 80% yield from inexpensive (C)- and ( )-camphor-10-sulfonic acids. Alternatively they are commercially available200.
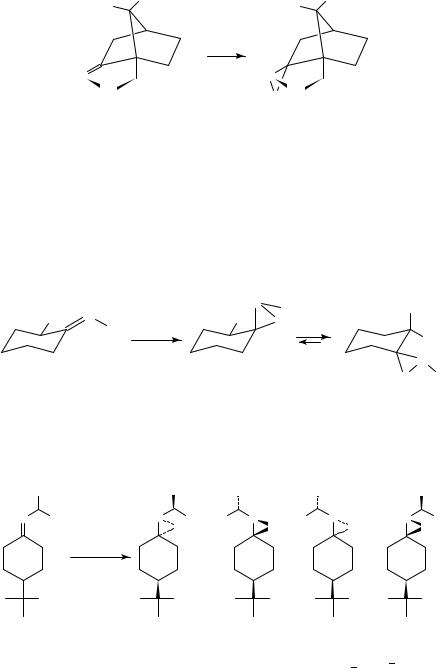
The oxidation of imines derived from substituted cyclohexanones occurs predominantly from the equatorial direction. However, the product oxaziridines can undergo subsequent equilibration to favor a more stable conformation which places the bulkier nitrogen substituent in an equatorial conformation (equation 44)219.
|
|
N |
Bn |
Me |
|
Me N |
Me |
|
|
||
|
O |
|
|
||
Bn |
MCPBA |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
O |
Bn |
44
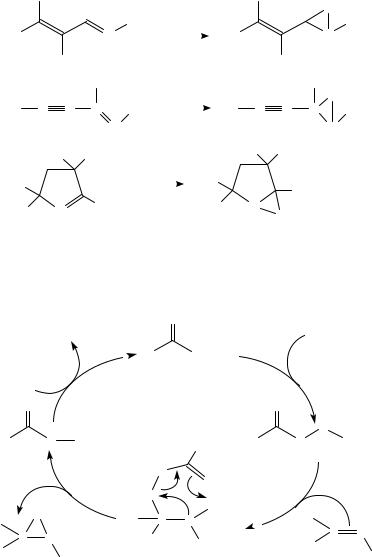
In another example, the cumulative effect of equatorial attack in prochiral cyclohexanoneimines with diastereoselectivity induced by a chiral nitrogen substituent allowed the synthesis of spirocyclic oxaziridines with a high induction of axial dissymmetry. The major oxaziridine isomer results from both the favored equatorial attack and oxidation anti to the chiral nitrogen substituent (equation 45)204
Me
N Ph
MCPBA or
(+) MPCA
85 %
(Ph)
Me |
|
Me |
Me |
|
Me |
N |
Ph |
Ph N |
O Ph |
N O |
N O Ph |
O |
|||||
|
|
+ |
+ |
|
+ |
(Ph) |
|
(Ph) |
(Ph) |
(Ph) |
|
74−85% 12−20% 3−5% 1−3% 45
On epoxidation of imines containing several oxidizable functions, significant selectivity was observed in favor of the formation of oxaziridines (equations 46 48)220 222.
|
|
20. Epoxidation of CDX double bonds |
|
1245 |
||||||||||
|
H |
|
|
|
|
|
|
|
|
|
H |
|
O |
|
|
|
|
But |
|
|
|
|
|
|
|
|
But |
||
|
|
|
MCPBA |
|
|
|
|
|
|
|||||
Ph |
|
N |
|
|
|
|
Ph |
|
|
N |
(46) |
|||
|
|
|
|
|
|
|
|
|
|
|
||||
|
H(Me) |
|
|
|
|
|
|
|
|
|
H(Me) |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
H |
O |
|
|
|
|
But |
|
MCPBA |
|
|
|
|
|
But (47) |
|||
Ph |
C C |
C |
|
|
|
Ph |
C |
C C |
|
|||||
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|||||||||
|
|
N |
|
|
|
|
|
|
|
|
|
|
N |
|
|
R |
OCOY |
|
|
|
|
|
|
|
|
R |
OCOY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Me |
|
|
|
MCPBA |
Me |
|
H |
|
(48) |
|||||
|
|
|
|
|
|
|
|
|
|
|||||
Me |
N |
H |
|
|
|
|
|
Me |
N |
|
|
|||
|
|
|
|
|
O |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
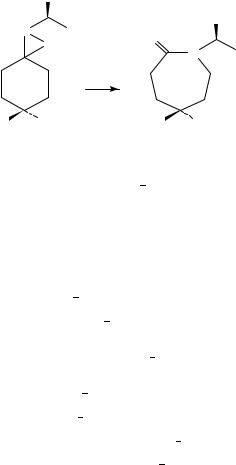
The metal-catalyzed oxidation of imines using molecular oxygen as the final oxidant and aldehydes as co-reductants has been studied223. Various transition metal complexes have been tested as catalysts and it is found that cobalt complexes can catalyze the selective oxidation of imines to oxaziridines in good yield (ca 80%) (Scheme 4).
|
|
|
O |
|
|
|
|
RCOOH |
|
|
O2 |
|
|
|
|
R |
CoIIICl2 |
|
|
|
RCHO |
|
|
|
|
|
|
O |
|
|
|
O |
|
|
|
O CoIIICl2 |
|
|
O |
CoIIICl2 |
|
R |
|
R |
O |
|||
|
|
|
R |
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
||
|
|
O |
CoIIICl2 |
|
|
|
O |
1 |
|
1 |
|
||
|
|
|
||||
R1 |
R |
C |
N |
R |
|
|
C |
N |
|||||
C |
N |
R2 |
R3 |
|||
R2 |
R3 |
|||||
R2 |
R3 |
|
|
|
||
|
|
|
|
|
SCHEME 4
C. Reactions of Oxaziridines
Considering that the present paper does not intend to analyze the chemical reactions of oxaziridines, we refer only to some publications in this field published in recent years. Certain oxaziridines undergo stereoelectronically controlled photochemical rearrangement into lactams (equation 49)204,219.
1246 |
Mihaly´ Bartok´ and Gyula Schneider |
|
|
Me |
|
|
|
Me |
N |
|
Ph |
|
O |
O |
N |
Ph |
(49) |
|
|
hν
R1 |
R2 |
R1 |
R2 |
Oxaziridines are synthetically useful reagents to transfer an oxygen to a variety of substrates. Within this, chiral oxaziridines5,200,202 attained especially great significance.
The research work of recent years includes predominantly the epoxidation of alkenes9,200, asymmetric hydroxylations209,224 228 and the asymmetric oxidation of sulfides to sulfoxides205,209,229,230. Optical yields of practical significance were obtained (>90%). A detailed review published in 1991231 reports about the versatile use of oxaziridines in the field of the electrophilic amination.
VI. REFERENCES
1.M. Bartok´ and K. L. Lang,´ in The Chemistry of Functional Groups, Supplement E (Ed. S. Patai), Wiley, Chichester, 1980, pp. 610 627.
2.M. Bartok´ and K. L. Lang,´ in The Chemistry of Heterocyclic Compounds (Ed. A. Hassner), Vol. 42, Wiley, New York, 1985, pp. 15 57.
3.R. W. Murray, Chem. Rev., 89, 1187 (1989).
4.M. J. Haddadin and J. P. Freeman, in The Chemistry of Heterocyclic Compounds (Ed. A. Hassner), Vol. 42, Chap. 3, Wiley, New York, 1985, pp. 283 350.
5.F. A. Davis and M. S. Haque, Adv. Oxygenated Processes, 2, 61 (1990).
6.A. S. Rao, in Comprehensive Organic Synthesis, Vol. 7 (Eds. B. M. Trost and I. Fleming), Pergamon Press, Oxford, 1991, pp. 357 387.
7.J. Aube, in Comprehensive Organic Synthesis, Vol. 1 (Eds. B. M. Trost and I. Fleming), Pergamon Press, Oxford, 1991, pp. 819 842.
8.R. A. Johnson and K. B. Sharpless, in Comprehensive Organic Synthesis, Vol. 7 (Eds. B. M. Trost and I. Fleming), Pergamon Press, Oxford, 1991, pp. 389 436.
9. Y. Sawaki, in The Chemistry of Hydroxyl, Ether and Peroxide groups, Supplement E2 (Ed.
S. Patai), Chap 11, Wiley, Chichester, 1993, pp. 588 621.
10.E. N. Jacobsen, in Catalytic. Asymmetric Syntheses (Ed. I. Ojima), VCH, New York, 159 (1993).
11.B. Plesnicar, V. Nemart, M. Hodoscek, J. Koller, F. Kovacic and J. Skerjanc, J. Chem. Soc., Perkin Trans. 2, 1397 (1986).
12.M. Oldani, T. K. Ha and A. Baulder, J. Am. Chem. Soc., 105, 360 (1983).
13.A. A. Shered’ko, Z. G. Pikh and E. N. Mokryi, Dokl. Akad. Nauk Ukr. SSR, Ser. B: Geol., Khim Biol. Nauki, 49 (1990).
14.A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 93, 1307 (1993).
15.A. Pfenninger, Synthesis, 89 (1985).
16.P. Kocovsky, Tetrahedron Lett., 29, 2475 (1988).
17.B. A. McKittrick and B. Ganem, Tetrahedron Lett., 26, 4895 (1985).
18.P. Kocovsky and I. Stary, J. Org. Chem., 55, 3236 (1990).
19.I. Honda A. Ori and G. Tsuchihashi, Chem. Lett., 1417 (1986).
20.E. A. Mash, Synlett, 529 (1991).
21.K. Mori, K. Fukamatsu and M. Kido, Justus Liebigs Ann. Chem., 657 (1993).
22.H. E. Schink, H. Petterson and J.-E. Backvall, J. Org. Chem., 56, 2769 (1991).
23.M. D. Threadgill and P. Webb, J. Chem. Soc., Chem. Commun., 269 (1991).
24.D. P. Rotella, Tetrahedron Lett., 30, 1913 (1989).
25.F. Mohamadi and M. M. Spees, Tetrahedron Lett., 30, 1309 (1989).

20. Epoxidation of CDX double bonds |
1247 |
26.K. Maruoka, R. Bureau and H. Yamamoto, Synlett, 363 (1991).
27.J. Rebek, Jr., L. Marshall, J. McManis and R. Wolak, J. Org. Chem., 51, 1649 (1986).
28.R. Nagata and I. Saito, Synlett, 291 (1990).
29.J. Mazur, Synthesis, 1325 (1995).
30.A. M. d’A. Rocha Gonsalves, R. A. W. Johnstone, M. M. Pereira and J. Shaw, J. Chem. Res.
(S), 208 (1991); (M), 2101 (1991).
31.W. W. Zajac, Jr., T. R. Walters and J. M. Woods, Synthesis, 808 (1988).
32.E. V. Dehmlow and B. Vehre, New. J. Chem., 13, 117 (1989).
33.G. Xie, L. Xu, J. Hu, S. Ma, W. Hou and F. Tao, Tetrahedron Lett., 29, 2967 (1988).
34.R. L. Safiullin, L. R. Enikeeva, S. Yu. Serenko, V. D. Komissarov and G. A. Tolstikov, Izv. Akad. Nauk SSSR, Ser. Khim., 333 (1991).
35.W. Zhu and W. T. Ford, J. Org. Chem., 56, 7022 (1991).
36.T. Kamiyama, M. Inoue, H. Kashiwagi and S. Enomoto, Bull. Chem. Soc. Jpn., 63, 1559 (1990).
37.Y. Ishii and M. Ogawa, in Hydrogen Peroxide Oxidation Catalyzed by Heteropoly Acids Combined with Cetylpyridinium Chloride, Vol. 3, MYU, Tokyo, 1990, p. 121.
38.C. Venturello and M. Gambaro, Synthesis, 295 (1989).
39.M. Quenard, V. Bonmarin, G. Gelbard and L. Krumenacker, New. J. Chem., 13, 183 (1989).
40.C. Aubry, G. Chottard, N. Platzer, J.-M. Bregeault,´ R. Thouvenot, F. Chauveau, C. Huet and H. Ledon, Inorg. Chem., 30, 4409 (1991).
41.C. Venturello, R. D’Aloiso, J. C. Bart and M. Ricci, J. Mol. Catal., 32, 107 (1985).
42.S. Sakue, T. Tsubakino, Y. Nishiyama and Y. Ishii, J. Org. Chem., 58, 3633 (1993).
43.Y. Ishii, H. Tanaka and Y. Nishiyama, Chem. Lett., 1 (1994).
44.D. C. Duncan, R. C. Chambers, E. Hecht and C. L. Hill, J. Am. Chem. Soc., 117, 681 (1995).
45.A. C. Dengel, W. P. Griffith and B. C. Parkin, J. Chem. Soc., Dalton Trans, 2683 (1993).
46.L. Sale, C. Aubry, F. Robert, G. Chottard, R. Thouvenot, H. Ledon and J.-M. Bregeault,´ New J. Chem., 17, 367 (1993).
47.R. Neumann and M. Gara, J. Am. Chem. Soc., 117, 5066 (1995).
48.K. A. Jorgensen, Chem. Rev., 89, 431 (1989).
49.H. J. Ledon, P. Durbat and F. Varescon, J. Am. Chem. Soc., 103, 3601 (1981).
50.H. Mimoun, in Comprehensive Coordination Chemistry, Vol. 6 (Ed. G. Wilkinson), Pergamon, New York, 1987, pp. 317 410.
51.D. Kaminski, O. N. Temkin and D. Bonchev, Appl. Catal. A, 88, 1 (1992).
52.M. T. Reetz and E. H. Lauterbach, Tetrahedron Lett., 32, 4477 (1991).
53.R. F. W. Jackson, S. P. Standen and W. Clegg, J. Chem. Soc., Perkin Trans. 1, 149 (1995).
54.T. J. Murry and J. T. Groves, in Cytochrome P-450, Structure, Mechanism and Biochemistry
(Ed. P. R. Ortiz de Montellano), Plenum Press, New York, 1986, p. 1.
55.P. W. White, Bioorg. Chem., 18, 440 (1990).
56.W. Nam and J. S. Valentine, J. Am. Chem. Soc., 112, 4977 (1990).
57.K. Yamaguchi, Y. Watanabe and I. Morishima, J. Chem. Soc., Chem. Commun., 1721 (1992).
58.J. P. Collman, X. Zhang, R. T. Hembre and J. I. Brauman, J. Am. Chem. Soc., 112, 5356 (1990).
59.J. P. Collman, P. D. Hampton and J. T. Brauman, J. Am. Chem. Soc., 112, 2977 (1990).
60.D. Mansuy, J. F. Bartoli, P. Battioni, D. K. Lyon and R. G. Finke, J. Am. Chem. Soc., 113, 7222 (1991).
61.W. Nam and J. S. Valentine, J. Am. Chem. Soc., 113, 7449 (1991).
62.T. Kim, G. A. Mirafzal, J. Liu and N. L. Bauld, J. Am. Chem. Soc., 115, 7653 (1993).
63.L. C. Yuan and T. C. Bruice, J. Am. Chem. Soc., 108, 1643 (1986).
64.R. D. Arasasingham, G.-X. He and T. C. Bruice, J. Am. Chem. Soc., 115, 7985 (1993).
65.R. W. Lee, P. C. Nakagaki and T. C. Bruice, J. Am. Chem. Soc., 111, 1368 (1989).
66.R. L. Halterman and S.-T. Jan, J. Org. Chem., 56, (1991).
67.A. W. Van der Made, R. J. M. Nolte and W. Drenth, Recl. Trav. Chim. Pays-Bas, 109, 537 (1990).
68.S. Banfi, M. Dragoni, F. Montanare, G. Pozzi and S. Quici, Gazz. Chim. Ital., 123, 431 (1993).
69.P. L. Anelli, S. Banfi, F. Legramandi, F. Montanari, G. Pozzi and S. Quici, J. Chem. Soc., Perkin Trans. 1, 1345 (1993).
70.S. Banfi, F. Montanari and S. Quici, Recl. Trav. Chim. Pays-Bas, 109, 117 (1990).
71.T. G. Traylor, S. Tsuchiya, Y. S. Byun and C. Kim, J. Am. Chem. Soc., 115, 2775 (1993).
72.D. D. Agarwal, R. Rastogi and L. Sharma, Indian J. Chem., Sect. B, 31B, 128 (1992).
73.K. Miki and Y. Sato, Bull. Chem. Soc. Jpn., 66, 2385 (1993).
1248 |
Mihaly´ Bartok´ and Gyula Schneider |
74.T. Hirao, M. Ohno and Y. Ohshiro, Tetrahedon Lett., 31, 6039 (1990).
75.H. Nishihara, K. Pressprich, R. W. Murray and J. P. Collman, Inorg. Chem., 29, 1000 (1990).
76.T. Higuchi, H. Ohtake and M. Hirobe, Tetrahedron Lett., 30, 6545 (1989).
77.S. Campestrini, A. Robert and B. Meunier, J. Org. Chem., 56, 3725 (1991).
78.T. G. Traylor and A. R. Miksztal, J. Am. Chem. Soc., 111, 7443 (1989).
79.D. Ostovic and T. C. Bruice, J. Am. Chem. Soc., 111, 6511 (1989).
80.D. Ostovic and T. C. Bruice, Acc. Chem. Res., 25, 314 (1992).
81.J. T. Groves and T. E. Nemo, J. Am. Chem. Soc., 105, 5786 (1983).
82.M. E. de Carvalho and B. Meunier, Nouv. J. Chim., 10, 223 (1986).
83.B. de Poorter and B. Meunier, J. Chem. Soc., Perkin Trans. 2, 1732 (1985).
84.Y. Naruta, F. Tani, N. Ishihara and K. Maruyama, J. Am. Chem. Soc., 113, 6865 (1991).
85.G. Proess and L. Hevesi, J. Mol. Catal., 80, 395 (1993).
86.S. O’Malley and T. Kodadek, J. Am. Chem. Soc., 111, 9116 (1989).
87.K. Konishi, K. I. Oda, K. Nishida, T. Aida and S. Inoue, J. Am. Chem. Soc., 114, 1313 (1992).
88.S. Inoue, T. Aida and K. Konishi, J. Mol. Catal., 74, 121 (1992).
89.Y. Kuroda, T. Hiroshiga and H. Ogoshi, J. Chem. Soc., Chem. Commun., 1594 (1990).
90.S. Banfi, F. Montanari, G. Pozzi and S. Quici, Gazz. Chim. Ital., 123, 617 (1993).
91.J. P. Collman, Z. Zhang, V. Lee, R. T. Hembre and J. I. Brauman, Adv. Chem. Ser., 230, 153 (1992).
92.J. P. Collman, X. Zhang, V. J. Lee and J. I. Brauman, J. Chem. Soc., Chem. Commun., 1647 (1992).
93.J. P. Collman, X. Zhang, J. A. Ibers and J. I. Brauman, J. Am. Chem. Soc., 115, 3834 (1993).
94.J. P. Collman, X. Zhang, V. J. Lee, E. Uffelman and J. I. Brauman, Science, 261, 1404 (1993).
95.J. P. Collman, V. J. Lee, C. J. Kellen-Yuen, X. Zhang, J. A. Ibers and J. I. Brauman, J. Am. Chem. Soc., 117, 692 (1995).
96.G. Legemaat, W. Drenth, M. Schmidt, G. Prescher and G. Goor, J. Mol. Catal., 62, 119 (1990).
97.T. G. Traylor, Y. S. Byun, P. S. Traylor, P. Battioni and D. Mansuy, J. Am. Chem. Soc., 113, 7821 (1991).
98.P. Hoffmann and B. Meunier, New J. Chem., 16, 559 (1992).
99.G. X. He, H. Y. Mei and T. C. Bruice, J. Am. Chem. Soc., 113, 5644 (1991).
100.W. Zhang and E. N. Jacobsen, J. Org. Chem., 56, 2296 (1991).
101.W. Zhang, J. L. Loebach, S. R. Wilson and E. N. Jacobsen, J. Am. Chem. Soc., 112, 2801 (1990).
102.Y. D. Wu and K. N. Houk, Chemtracts: Org. Chem., 3, 350 (1990).
103.R. Irie, Y. Ito and T. Katsuki, Synlett, 265 (1991).
104.R. Irie, K. Noda, Y. Ito, N. Matsumoto and T. Katsuki, Tetrahedron: Asymmetry, 2, 481 (1991).
105.N. Hosoya, R. Irie, Y. Ito and T. Katsuki, Synlett, 691 (1991).
106.N. Hosoya, A. Hatayama, K. Yanai, H. Fujii, R. Irie and T. Katsuki, Synlett, 641 (1993).
107.T. Schwenkreis and A. Berkessel, Tetrahedron Lett., 34, 4785 (1993).
108.B. B. De, B. B. Lohray and P. K. Dhal, Tetrahedron Lett., 34, 2371 (1993).
109.N. H. Lee and E. N. Jacobsen, Tetrahedron Lett., 32, 6533 (1991).
110.E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker and L. Deng, J. Am. Chem. Soc., 113, 7063 (1991).
111.N. Hosoya, A. Hatayama, R. Irie, H. Sasaki and T. Katsuki, Tetrahedron, 50, 4311 (1994).
112.T. Yamada, K. Imagawa, T. Nagata and T. Mukaiyama, Chem. Lett., 2231 (1992).
113.K. Imagawa, T. Nagata, T. Yamada and T. Mukaiyama, Chem. Lett., 527 (1994).
114.T. Hamada, R. Irie and T. Katsuki, Synlett, 479 (1994).
115.R. Irie, K. Noda, Y. Ito, N. Matsumoto and T. Katsuki, Tetrahedron Lett., 31, 7345 (1990).
116.R. Irie, K. Noda, Y. Ito and T. Katsuki, Tetrahedron Lett., 32, 1055 (1991).
117.H. Sasaki, R. Irie and T. Katsuki, Synlett, 300 (1993).
118.H. Sasaki, R. Irie, T. Hamada, K. Suzuki and T. Katsuki, Tetrahedron, 41, 1187 (1994).
119.N. Hosoya, R. Irie and T. Katsuki, Synlett, 261 (1993).
120.T. Hayashi, F. Ozawa and Y. Kobatake, Kagaku (Kyoto), 47, 564 (1992); Chem. Abstr., 117, 191019e (1992).
121.T. Mukaiyama, T. Yamada, T. Nagata and K. Imagawa, Chem. Lett., 2, 327 (1993).
122.R. L. Halterman and T. M. Ramsey, Organometallics, 12, 2879 (1993).
123.S. L. Colletti and R. L. Halterman, J. Organomet. Chem., 455, 99 (1993).
124.T. Nagata, K. Imagawa, T. Yamada and T. Mukaiyama, Chem. Lett., 1259 (1994).
125.T. Nagata, K. Imagawa, T. Yamada and T. Mukaiyama, Inorg. Chim. Acta, 220, 283 (1994).

20. Epoxidation of CDX double bonds |
1249 |
126.C. Bolm, Angew. Chem., 103, 414 (1991).
127.Yu. E. Raifel’d and A. M. Vaisman, Usp. Khim., 60, 241 (1991).
128.B. Meunier, Chemtracts: Inorg. Chem., 3, 347 (1991).
129.V. Schurig and F. Betschinger, Chem. Rev., 92, 873 (1992).
130.R. Irie, Yuki Gosei Kagaku Kyokaishi, 51, 412 (1993); Chem. Abstr., 119, 94684b (1993).
131.E. Hoeft, Top. Curr. Chem., 164, 63 (1993); Chem. Abstr., 119, 203243e (1993).
132.T. Katsuki, Kagaku to Seibutsu, 31, 689 (1993); Chem. Abstr., 120, 106038e (1994).
133.E. Manoury, H. A. H. Moulard and G. G. A. Balavoine, Tetrahedron: Asymmetry, 4, 2339 (1993).
134.G. Balavoine, N. Crenne and E. Manoury, Fr. Pat. 2,638,162 (1988); Chem. Abstr., 114, 6812k (1991).
135.W. P. S. Shum, J. G. Zajacek and H. S. Kesling, Eur. Pat. 472,790 (1990); Chem. Abstr., 116, 214324w (1992).
136.W. P. Shum and H. S. Kesling, Jr., U. S. Pat. 5,103,027 (1991); Chem. Abstr., 116, 255463j (1992).
137.J. Rodriguez and J. P. Dulcere, J. Org. Chem., 56, 469 (1991).
138.N. L. Bauld and G. A. Mirafzal, J. Am. Chem. Soc., 113, 3613 (1991).
139.Y. Masaki, T. Miura, I. Mukai, A. Itoh and H. Oda, Chem. Lett., 1937 (1991).
140.M. G. Clerici and P. Ingallina, J. Catal., 140, 71 (1993).
141.E. Bosch and J. K. Kochi, J. Chem. Soc., Chem. Commun., 667 (1993).
142.C. Potvin, E. Duprey, O. Mohammedi and J. M. Bregeault, C. R. Acad. Sci. Paris, Ser. II., 317, 757 (1993); Chem. Abstr., 120, 323107g (1994).
143.T. Takai, E. Hata, T. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 64, 2513 (1991).
144.M. J. Upadhyay, P. K. Bhattacharya, P. A. Ganeshpure and S. Satish, J. Mol. Catal., 73, 277 (1992).
145.T. Takai, E. Hata, K. Yorozu and T. Mukaiyama, Chem. Lett., 2077 (1992).
146.E. Bouhlel, P. Laszlo, M. Levart, M. T. Montaufier and G. P. Singh, Tetrahedron Lett., 34, 1123 (1993).
147.T. Tanase, K. Mano and Y. Yamamoto, Inorg. Chem., 32, 3995 (1993).
148.I. Yamakawa, H. Urabe, Y. Kobayashi and F. Sato, Tetrahedron Lett., 32, 2043 (1991).
149.S. Takano, Y. Iwabuchi and K. Ogasawara, Tetrahedron Lett., 32, 3527 (1991).
150.K. Mori and S. Harashima, Justus Liebigs Ann. Chem., 391 (1993).
151.J. Gay and G. Scherowsky, Synth. Commun., 25, 2665 (1995).
152.A. D. Westwell, M. Thornton-Pett and C. M. Rayner, J. Chem. Soc., Perkin Trans. 1, 847 (1995).
153.P. Besse and H. Verschambre, Tetrahedron, 50, 8885 (1994).
154.M. M. T. Khan and R. S. Shukla, J. Mol. Catal., 72, 361 (1992).
155.E. J. Allain, L. P. Hager, L. Deng and E. N. Jacobson, J. Am. Chem. Soc., 115, 4415 (1993).
156.I. Okura, Jpn. Kokai 02 92,295 (1988); Chem. Abstr., 113, 113887q (1990).
157.S. W. May, M. S. Steltenkamp, R. P. Schwartz and C. J. McCoy, J. Am. Chem. Soc., 98, 7856 (1976).
158.H. Fu, G. J. Shen and C. H. Wong, Recl. Trav. Chim. Pay-Bas, 110, 167 (1991).
159.N. R. Woods and J. C. Murrell, Biotechnol. Lett., 12, 409 (1990).
160.M. Mahmoudian and A. Michael, Appl. Microbiol. Biotechnol., 37, 23 (1992).
161.M. Mahmoudian and A. Michael, Appl. Microbiol. Biotechnol., 37, 28 (1992).
162.O. Takahashi, J. Umezawa, K. Furuhashi and M. Takagi, Tetrahedron Lett., 30, 1583 (1989).
163.H. Kropf, in Methoden der Organischen Chemie (Houben-Weyl), Bd. E 13/1, Georg Thieme Verlag, New York, 1988, pp. 618 619.
164.W. Adam, in The Chemistry of Functional Groups, Peroxides (Ed. S. Patai), Chap. 24, Wiley, Chichester, 1983.
165.A. Baeyer and V. Villiger, Chem. Ber., 32, 3625 (1899).
166.R. E. Montgomery, J. Am. Chem. Soc., 96, 7820 (1974).
167.W. Adam, R. Curci and J. O. Edwards, Acc. Chem. Res., 22, 205 (1989).
168.R. Curci, Adv. Oxygenated Processes, 2, 1 (1990).
169.W. Adam, L. P. Hadjiarapoglou, R. Curci and R. Mello, Org. Peroxides, 195 (1992).
170.R. W. Murray and R. J. Jeyaraman, J. Org. Chem., 50, 2847 (1985).
171.R. Mello, M. Fiorentino, O. Sciacovelli and R. Curci, J. Org. Chem., 53, 3890 (1988).
172.W. Adam, L. Hadjiarapoglou and A. Smerz, Chem. Ber., 124, 227 (1991).
173.R. W. Murray, M. Singh and R. Jeyaraman, J. Am. Chem. Soc., 114, 1346 (1992).
174.A. L. Baumstark and P. C. Vasquez, J. Org. Chem., 53, 3437 (1988).
1250 |
Mihaly´ Bartok´ and Gyula Schneider |
175.R. W. Murray and D. L. Shiang, J. Chem. Soc., Perkin Trans 2, 349 (1990).
176.R. W. Murray and D. Gu, J. Chem. Soc., Perkin Trans 2, 2203 (1993).
177.A. M. Lluch, F. Sanchez-Baeza, A. Messeguer, C. Fusco and R. Curci, Tetrahedron, 49, 6299 (1993).
178.C. Wameling, H. R. Glatt, F. Oesch and A. Seidel, Polycyclic Aromat. Compd., 3, 191 (1993); Chem. Abstr., 121, 157441j (1994).
179.R. W. Murray and W. Kong, Polycyclic Aromat. Compd., 5, 139 (1994); Chem. Abstr., 121, 307098u (1994).
180.W. Adam, M. Sauter and C. Zuenkler, Chem. Ber., 127, 1115 (1994).
181.W. Adam, L. Hadjiarapoglou, K. Mielke and A. Treiber, Tetrahedron Lett., 35, 5625 (1994).
182.R. Curci, A. Detomaso, T. Prencipe and G. B. Carpenter, J. Am. Chem. Soc., 116, 8112 (1994).
183.F. Nan, X. Chen, Z. Xiong, T. Li and Y. Li, Chin. Chem. Lett., 5, 15 (1994); Chem. Abstr., 121, 35215t (1994).
184.W. Adam, J. Halasz, A. Levai, Cs. Nemes, T. Patonay and G. Toth, Justus Liebigs Ann. Chem., 795 (1994).
185.P. Bovicelli, P. Lupattelli and E. Mincione, J. Org. Chem., 59, 4304 (1994).
186.A. Mukherjee and W. C. Agosta, Chemtracts: Org. Chem., 7, 102 (1994).
187.M. A. Maestro, A. Mourino, B. Borsje and S. J. Halkes, Can. Pat. 2,104,418 (1992); Chem. Abstr., 121, 108057e (1994).
188.D. Kuck, A. Schuster, C. Fusco, M. Fiorentino and R. Curci, J. Am. Chem. Soc., 116, 2375 (1994).
189.W. Adam and F. Prechtl, Chem. Ber., 127, 667 (1994).
190.R. W. Murray and D. Gu, J. Chem. Soc., Perkin Trans. 2, 451 (1994).
191.A. L. Baumstark, M. Beeson and P. C. Vasquez, Tetrahedron Lett., 30, 5567 (1989).
192.R. Curci, L. D’Accolti, A. Detomaso, C. Fusco, K. Takeuchi, Y. Ohga, P. E. Eaton and Y. C. Yip,
Tetrahedron Lett., 34, 4559 (1993).
193.A. Altamura, R. Curci and J. O. Edwards, J. Org. Chem., 58, 7289 (1993).
194.W. Adam and M. Sauter, Tetrahedron, 50, 8393 (1994).
195.J. K. Crandall and T. Reix, Tetrahedron Lett., 35, 2513 (1994).
196.M. Ferrer, F. Sanchez-Baeza, J. Casas and A. Messeguer, Tetrahedron Lett., 35, 2981 (1994).
197.W. D. Emmons, J. Am. Chem. Soc., 78, 6208 (1956).
198.W. D. Emmons, J. Am. Chem. Soc., 79, 5739 (1957).
199.P. M. Henry and G. L. Lange, in The Chemistry of Double-bonded Functional Groups. Supplement A2 (Ed. S. Patai), Chap. 11, Wiley, London, 1977.
200.F. A. Davis and A. C. Sheppard, Tetrahedron, 45, 5703 (1989).
201.F. A. Davis and R. H. Jenkins Jr., in Asymmetric Synthesis, Vol. 4 (Ed. J. D. Morrison), Chap. 4, Academic Press, New York, 1984.
202.F. A. Davis and B.-C. Chen, Chem. Rev., 92, 919 (1992).
203.L. C. Vishwakarma, O. D. Stringer and F. A. Davis, Org. Synth., 66, 203 (1988).
204.J. Aube,´ Y. Wang, M. Hammond, M. Tanol, F. Takusagawa and D. Van der Velde, J. Am. Chem. Soc., 112, 4879 (1990).
205.F. A. Davis, R. T. Reddy, W. Han and P. J. Carroll, J. Am. Chem. Soc., 114, 1428 (1992).
206.F. A. Davis, S. Chattopadhyay, J. C. Towson, S. Lal and T. Reddy, J. Org. Chem., 53, 2087 (1988).
207.J. C. Towson, M. C. Weismiller, G. S. Lal, A. C. Sheppard and F. A. Davis, Org. Synth., 69, 158 (1990).
208.D. R. Boyd, P. B. Coulter, M. R. McGuckin, N. D. Sharma, W. B. Jennings and V. E. Wilson,
J. Chem. Soc., Perkin Trans. 1, 301 (1990).
209.F. A. Davis, M. C. Weismiller, C. K. Murphy, R. T. Reddy and B.-C. Chen, J. Org. Chem., 57, 7274 (1992).
210.A. R. Hajipour and S. G. Pyne, J. Chem. Res., Synop., 388 (1992).
211.A. Azman, J. Koller and B. Plesnicar, J. Am. Chem. Soc., 101, 1107 (1979).
212.V. A. Petrov, D. D. Desmarteau and W. Navarrini, Eur. Pat. 496,413 (1991); Chem. Abstr., 117, 191831p (1992).
213.V. A. Petrov and D. D. DesMarteau, J. Org. Chem., 58, 4754 (1993).
214.W. Navarrini and D. D. DesMarteau, U. S. Pat. 4,874,875; Chem. Abstr., 112, 159140y (1990).
215.L. Bragante and D. D. DesMarteau, J. Fluorine Chem., 53, 181 (1991).
216.A. Bulachkova, G. I. Koldobskii, G. F. Tereshchenko, A. G. Drozdetskii and L. A. Bagrievich, SU Pat. 1,616,914 (1988); Chem. Abstr., 115, 8773t (1991).
20. Epoxidation of CDX double bonds |
1251 |
217.V. A. Petrov, D. D. Desmarteau and L. Bragante, Eur. Pat. 496,414 (1991); Chem. Abstr., 117, 191830n (1992).
218.V. A. Petrov and D. D. DesMarteau, Mendeleev Commun., 87 (1993).
219.J. Aube,´ M. Hammond, E. Gherardini and F. Takusagawa, J. Org. Chem., 56, 499 (1991).
220.S. B. Said, J. Mlochowski and J. Skarzewski, Justus Liebigs Ann. Chem., 5, 461 (1990).
221.J. Mlochowski, F. F. Abdel-Latif, E. Kubicz and S. B. Said, Pol. J. Chem., 67, 711 (1993).
222.N. J. Gibson and A. R. Forrester, J. Chem. Soc., Perkin Trans. 1, 491 (1995).
223.L. Martiny and K. A. Jorgensen, J. Chem. Soc., Perkin Trans. 1, 699 (1995).
224.F. A. Davis, A. Kumar and B.-C. Chen, J. Org. Chem., 56, 1143 (1991).
225.F. A. Davis, A. Kumar, R. E. Reddy, B.-C. Chen, P. A. Wade and S. W. Shah, J. Org. Chem., 58, 7591 (1993).
226.W. Adam and F. Prechtl, Chem. Ber., 127, 667 (1994).
227.A. Arnone, M. Cavicchioli, V. Montanari and G. Resnati, J. Org. Chem., 59, 5511 (1994).
228.F. A. Davis, C. Clark, A. Kumar and B.-C. Chen, J. Org. Chem., 59, 1184 (1994).
229.R. D. Bach, B. A. Coddens, J. J. W. McDouall, H. B. Schlegel and F. A. Davis, J. Org. Chem., 55, 3325 (1990).
230.D. D. DesMarteau, V. A. Petrov, V. Montanari, M. Pregnolato and G. Resnati, J. Org. Chem., 59, 2762 (1994).
231.S. Andreae and E. Schmitz, Synthesis, 327 (1991).
