
20. Epoxidation of CDX double bonds |
1233 |
O
(15)
5c, A rIO
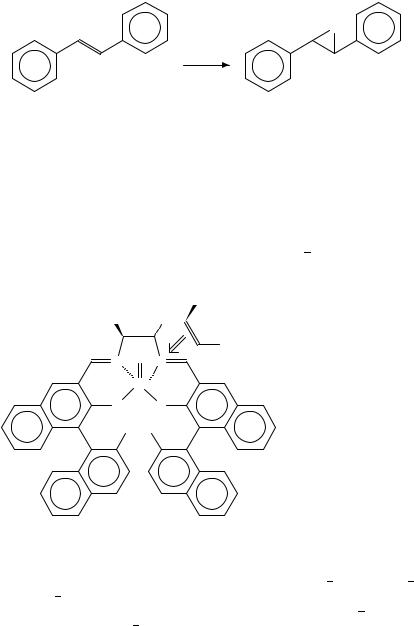
Recently, Katsuki and coworkers found that the epoxidation of cis-olefins conjugated with alkenyl, alkynil or aryl groups occurs with high enantioselectivity114. This interesting substrate-specific enentioselectivity observed in salen-catalyzed epoxidation can be explained by the newly proposed pathway a for the olefin access to metal oxo species (6). Two factors, repulsive steric and electronic interactions between the oncoming olefin and the salen ligand, are considered to be responsible for induction of asymmetry: the steric repulsion between the C-30 substituent in the salen ligand and an olefinic substituent and, -electronic repulsive interaction between the salen benzene ring (A) and the olefinic substituent bearing -bond direct a bulkier and more electron-rich olefinic substituent (L) away from the C-30 substituent. On the basis of the newly proposed hypothesis on the mechanism of asymmetric induction, a highly efficient (salen) manganese(III) complex
(6) was constructed as a catalyst for asymmetric epoxidation115 119.
|
|
L |
R |
R |
|
1′ |
2 ′ |
S |
|
a |
|
|
|
|
N |
O N |
|
L = alkenyl, alkynyl or aryl group S = alkyl group
8 1 |
+ |
1′ 8 ′ |
|
Mn |
|
O |
O |
A |
3 |
X X |
3 ′ |
(a) R = Ph, X = Me
(b) R = Ph, X = Ph
(c) R = 3,5 (CH3 )2 C6 H3 , X = Ph
AcO−
(6)
In addition to the above examples the significance of chiral epoxidation is manifested
by the appearance of numerous other results (for example, papers120 125, reviews126 133 and patents134 136).
Among others, new methods for epoxidation have been developed137 142 and new transition metal complexes143 147 have also been used.
4. Stereoselective and asymmetric epoxidation of allylic alcohols
Stereoselective and enantioselective epoxidations have been treated in detail in recent reviews8,9. A typical example is the stereoselective epoxidation of allylic and homoallylic

1234 |
Mihaly´ Bartok´ and Gyula Schneider |
alcohols8,9 on vanadium catalysts. The high syn selectivity of the vanadium catalyst is due to its strong coordination to the hydroxyl group (equations 16 and 17).
|
Me |
|
|
|
|
Me |
|
|
|
|
|
VO(acac)2 |
|
|
O |
OH (16) |
|
Me |
OH |
|
|
|
Me |
|
||
t-BuOOH |
|
|
||||||
|
|
|
|
|
|
|
||
|
Me |
|
|
|
|
Me Regioselection 49:1 |
||
|
OH |
|
|
|
|
OH |
|
|
|
Me |
|
|
|
|
Me |
|
|
|
|
VO(acac)2 |
|
|
|
O |
(17) |
|
Me |
|
t-BuOOH |
Me |
|
||||
|
|
|
||||||
|
Me |
|
|
|
Me |
Regioselection 20:1 |
|
|
Katsuki and Sharpless reported the new process of asymmetric epoxidation8 using a complex of titanium tetraisopropoxide and diethyl tartrate (DET), and t-butyl hydroperoxide (equation 18).
R |
R |
|
R |
O |
R |
Ti(OPri)4 /(+)-DET |
|
||||
|
|
|
|
(18) |
|
|
|
t-BuOOH |
|
|
|
R |
OH |
R |
|
OH |
|
|
|
|
|
The direction of the attack depends on the diethyl tartrate used in the reaction. The stereochemistry of the epoxidation, therefore, can be correctly predicted (equation 19).
|
|
|
R2 |
R1 |
|
|
|
O |
|
R2 |
R1 |
|
R3 |
OH |
|
i |
(−)-DET |
|
|
|
Ti(OPr )4 |
|
(19) |
|
|
t-BuOOH |
(+)-DET |
|
|
|
R2 |
R1 |
||
|
OH |
|
R3
O
OH
R3
This epoxidation is one of the most useful asymmetric reactions available to the synthetic chemist. The results show that, irrespective of the substitution pattern around the starting allylic alcohol, very good yields and excellent enantiomeric excesses are obtained148 152.
C. Biological Epoxidations
Epoxides are involved in the metabolism of many aliphatic and aromatic compounds in plants as well as in mammals. Enzyme potentiality allows both regioand stereospecific

20. Epoxidation of CDX double bonds |
1235 |
oxygenation reactions, which are very difficult to carry out chemically. The use of enzymes for such reactions is one of the most fascinating applications in bioconversion153 156.
Monooxygenases can activate molecular oxygen incorporating one oxygen atom into the substrate and reducing the other to water. In this way alkenes can be converted into epoxides (equation 20).
|
|
|
|
|
|
|
Monooxygenase |
H |
O |
H |
|
|
|
|
|
|
|
|
|||
R1 |
|
CH |
|
CH |
|
R2 |
O2 |
|
C C |
(20) |
|
|
|
|
|
||||||
|
|
|
|
|||||||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
R1 |
|
R2 |
Monooxygenases are found in many living organisms: bacteria, yeasts, insects, plants and mammal tissues. They are used for organic asymmetric reactions either in a more or less purified enzymatic form (cytochromes P-450) or in whole-cell microorganisms (bacteria, fungi).
1,7-Octadiene, which does not contain a terminal methyl group, is selectively converted by Pseudomonas oleovorans to the monoepoxide with an enantiomeric excess greater than 80%157 (equation 21).
O
CH2 |
|
CH |
|
(CH2 )4 |
|
CH |
|
CH2 |
|
(21) |
|
|
|
||||||||
|
|
|
|
|
(CH2 )4 CH CH2
Results show that structural limiting factors exist and that the enzymatic systems do not act on every substrate; thus they act on O-alkylated derivatives158, but not on allylic alcohols159. The epoxide products are generally of the R configuration. The epoxidation by Rhodococcus rhodochrous showed that the epoxidation rate and enantioselectivity of gaseous alkenes are very high159. Different genera of bacteria were tested utilizing ethene, propene or butenes160,161. The Corynebacterium equi was grown in an inorganic medium containing 1-hexadecene as the sole source of carbon162. The epoxidation of linear alkenes with carbon chains longer than 14 and with a terminal double bond proceeds stereospecifically. For example 1-hexadecene gives (R)-(+)-1,2-epoxyhexadecane with 100% enantiomeric excess (equation 22).
|
|
|
|
|
|
n-C14 H2 9 |
O |
n-C14 H2 9 |
|
CH |
|
CH2 |
|
(22) |
|
|
|
|
H |
||||
|
|
|
|||||
|
|
|
|||||
|
|
|
|
|
|
|
|
IV. EPOXIDATION OF THE C=O BOND |
|
||||||
A. Introduction
The epoxidation of ketones, i.e. the preparation of three-membered cyclic peroxides (dioxiranes), was developed in the 1980s. This field is so new that monographs dealing with the chemistry of peroxides do not mention in yet163,164.
As early as 1899 Baeyer and Villiger165 postulated a dioxirane intermediate in the KHSO5 oxidation of menthone to its lactone (equation 23). The first real contribution to dioxirane chemistry was made Montgomery166. Careful studies on the decomposition of the caroate ion permitted him to observe the acceleration of the reaction by the addition

1236 |
|
Mihaly´ Bartok´ and Gyula Schneider |
|
|
of acetone in neutral aqueous solutions. |
|
|
||
|
Me |
|
Me |
Me |
|
|
|
||
|
+ |
HOOSO2 OH |
|
O |
|
O |
|
||
|
|
|
|
|
|
O |
|
O |
O |
|
|
|
||
Me |
Me |
Me |
Me |
Me |
Me |
||||
23
The main achievements of dioxirane chemistry can be attributed to the activities of Curci, Edwards, Murray and their coworkers3,167. As a result of their detailed, systematic research, dioxiranes have become indispensable reagents in synthetic organic chemistry.
In addition to the review papers written by the leading experts of the field3,167 other reviews were also published in recent years9,168,169. Thus, we give only a short summary of dioxirane chemistry, referring, in the majority of cases, to the results published in the last 2 3 years.
|
60 |
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
|
|
H |
C |
+ O |
O− |
|
|
|
|
|
|
|
|
|
|
|||
|
23 |
H |
|
|
|
|
|
|
) |
|
22.8 |
|
|
|
|
||
−1 |
|
|
|
|
|
|
|
|
mol |
|
|
|
|
|
|
|
40 |
(kcal |
|
|
|
|
|
|
|
|
|
• |
|
|
|
|
|
|
|
energy |
H |
O |
O• |
|
|
|
|
|
|
C |
|
|
|
|
|
||
|
H |
|
|
|
|
|
|
|
Relative |
|
|
|
|
|
|
|
|
|
|
|
31.3 |
|
0.5 |
20 |
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
C |
O• |
|
|
|
|
|
15 |
H |
O• |
|
|
0 |
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H C O |
|
|
|
|
FIGURE 1. Energy diagram of the H2CO2 entities167

20. Epoxidation of CDX double bonds |
1237 |
The three-membered dioxirane ring is a strained system. In regard to the thermochemistry of these compounds, a number of theoretical analyses of the H2CO2 entity have been performed. The stability conditions are well demonstrated by the energy diagram in Figure 1.
B. Preparation of Dioxiranes
Epoxidation of ketones is carried out by the oxone reagent. The actual oxidizing agent is the caroate ion (equation 24).
R |
R |
O− |
|
C O + −O O SO2 |
O− |
C |
|
R′ |
R′ |
O O SO2 |
O− |
|
|
−SO4 2 − |
(24) |
|
R |
O |
|
|
|
C |
|
|
R′ |
O |
|
Numerous variations of this method were developed170 173. In general, 0.1 0.8 molar solutions of dioxiranes were prepared and used as oxidizing agents. These solutions can be stored in a refrigerator. Epoxidation of many ketones has been carried out in the above way, and the dioxiranes (7 14) thus prepared were characterized spectroscopically and also by chemical methods.
Me |
O |
Me |
O |
O |
|
|
O |
Me |
O |
F3 C |
O |
O |
|
Me |
O |
|
|
|
|||||
(7) |
|
(8) |
|
(9) |
|
(10) |
|
Me |
O |
Me |
|
O |
|
Me |
O |
|
|
|
|
|
O |
|
|
i-Pr |
O |
t-Bu |
|
O |
O |
Et |
O |
|
(11) |
|
(12) |
(13) |
|
(14) |
|
Dimethyldioxirane (7) and methyl(trifluoromethyl)dioxirane (8) are the two most effective reagents mainly used in preparative organic chemistry.

1238 |
Mihaly´ Bartok´ and Gyula Schneider |
C. Epoxidations by Dioxiranes
Dioxiranes can be used for the oxidation of various organic functional groups. The epoxidation of the olefinic double bond and the formation of the hydroxyl group through the oxidation of the C H bond have been studied most.
Dioxiranes epoxidize different compounds with CDC stereospecifically and electrophilically (equation 25) in high yield (90 100%).
≠
|
|
|
H |
a |
− |
H |
a |
O |
|
δ+ |
Oδ |
|
+ O |
C R′ |
|
O |
C R′ |
|
|
|
|||
|
|
|
a |
H |
|
a |
H |
R |
|
||
|
|
R |
|||
|
|
|
|
|
(25)
H |
a |
|
O |
|
|
|
|
|
O |
+ |
C |
|
|
|
|
a |
H |
R |
R′ |
A kinetic study of the epoxidation showed the reaction to be of the first order with respect to both alkene and dioxirane174. A large steric effect was observed in the epoxidation of certain cis/trans-dialkylalkenes; the cis compounds were found to exhibit reactivities one order of magnitude higher than the corresponding trans isomers. This large effect reflects a repulsive interaction between the substituents of the olefin and the dioxirane in the transition state (equation 25)9.
Several characteristic recent examples describing the epoxidation of ˛,ˇ-unsaturated ketones (equations 26 28) and that of ˛,ˇ-unsaturated carboxylic esters (equations 29 and 30) are given below.
|
O |
|
|
O |
|
|
|
O |
|
(26)173 |
|
Me |
+ |
Me |
O |
||
|
|||||
|
O |
|
|
||
Me |
Me |
Me |
Me |
||
|
O |
|
O |
|
|
|
Me |
O |
|
O |
|
|
+ |
|
|
(27)172 |
|
|
|
|
|
||
|
Me |
O |
|
|

|
|
20. Epoxidation of CDX double bonds |
|
1239 |
||||
R1 |
|
|
|
|
|
|
|
|
|
O |
H |
|
|
|
Me |
O |
|
|
|
|
|
|
|
|
||
R |
C |
C |
|
|
+ |
|
|
|
|
|
C |
|
R2 |
|
Me |
O |
|
|
|
H |
|
|
|
|
|
(28)172 |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
H |
|
|
|
|
|
R |
|
C |
C |
O |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
C |
|
R2 |
|
|
|
|
|
R1 |
H |
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
COOEt |
|
|
|
|
O |
|
|
O |
|
|
H |
COOEt |
|||
C |
C |
|
Me |
|
|
|||
|
|
|
C |
C |
||||
|
|
H |
+ |
|
|
|
||
|
|
|
|
|
|
H |
||
X |
|
|
Me |
O |
|
|
|
|
|
|
|
|
|
X |
|
(29)175,176 |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
CF3 |
|
Me |
|
Me |
|
|
|
|
Me |
|
|
|
|
CO2 Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
O |
R = Me, CF3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
O |
|
|
|
|
|
|
CF3 |
|
Me |
|
Me |
|
|
|
(30)177 |
|
|
|
|
|
CO2 Me |
|
||
Me |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
85% |
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
CF3 |
|
Me |
|
Me |
|
|
|
|
Me |
|
|
|
|
CO2 Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
|
|
|
|
|
15% |
|
|
|
|
|
|

1240 |
Mihaly´ Bartok´ and Gyula Schneider |
Several new examples for stereospecific epoxidation of compounds with more complicated structures can be seen in equations 31 38.
Me O
 OH +
OH +
Me O
OH
 O
O
|
|
|
OH |
R1 |
Me |
|
|
|
|
|
|
R2 |
|
Me |
R2 |
|
|
O |
|
|
Me |
+ |
|
|
O |
Me |
O |
R3 |
|
R3 |
|
R4 |
|
|
|
+ |
|
|
+ |
N2 |
|
|
N2 |
− |
Me |
O |
Me |
|
Me |
O |
|
|
|
||
|
|
|
O |
O |
|
|
O |
N2
Me O
Me O
O
OH
R1
R4
Me
O−
(31)178,179
Me
O
Me
O
(32)180
(33)181
O
O
O O
70%

|
|
20. Epoxidation of CDX double bonds |
|
|
1241 |
|||||||
|
Me |
|
|
|
|
|
|
|
|
Me |
|
|
|
Me |
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
Me |
|
|
|
|
|
|
Me |
|
|
H |
|
|
H |
Me |
|
O |
|
|
H |
|
H |
|
|
Me |
|
|
|
O |
Me |
|||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
F3 C |
|
O |
H |
|
|
|
(34)182 |
|
|
|
|
|
|
95% |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
RO |
|
|
|
|
|
|
RO |
|
|
|
|
|
Me |
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
Me |
O |
|
|
|
|
|
|
|
|
|
|
|
Me |
O |
|
|
|
|
|
|
|
(35)183 |
O |
Me |
|
|
|
|
|
O |
|
Me |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|||
Me |
Me |
|
|
|
Me |
|
Me |
|
|
|||
|
|
|
|
|
|
|
|
|||||
O |
|
|
|
R |
|
|
|
|
O |
|
|
R |
|
|
|
|
|
Me |
O |
|
|
|
|
|
|
|
|
|
|
|
Me |
O |
|
|
|
|
|
(36)184 |
|
|
|
|
|
|
|
|
|
|
O |
|
|
O |
H |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
H |
|
|
|||
|
|
Me |
O |
|
|
|
|
|
|
|
Me |
O |
O |
|
|
|
|
|
|
|
O |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
Me |
|
|
|
|
Me |
O |
|
O |
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
Me |
O |
|
|
|
|
|
(37)185 |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
Me |
O |
|
|
|
|
|
|
|
Me |
O |
|
|
|
|
|
|
|
|
|
|
|
||
Me |
|
|
|
|
Me |
O |
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
Me |
O |
|
|
|
|
|
(38)186 |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
Beside the preparation of epoxides and the formation of OH group186 190, dioxiranes can also be used to oxidize other functional groups191 196.

1242 |
Mihaly´ Bartok´ and Gyula Schneider |
Recent results clearly indicate that synthetic organic chemistry obtained a new, significant oxidizing agent by the epoxidation of ketones. Due to their high reactivity, regioand stereoselectivity, the use of neutral conditions in their synthetic applications and the easy product recovery, dioxiranes are unique oxidizing agents. The intensive utilization of dioxiranes as versatile oxidizing agents, however, has just started.
V. EPOXIDATION OF THE C=N BOND
A. Introduction
The epoxidation reaction of imines with peroxy acids was discovered by Emmons in 1956197,198. The reaction gave oxaziridines in good yield (equation 39).
R |
|
R |
|
|
C N R2 + RCO3 H |
|
C |
N R2 |
(39) |
|
||||
(H)R1 |
|
(H)R1 |
O |
|
Through the research work of the 40 years since the discovery, the chemistry of oxaziridines has become well known as earlier4,7,199 and recent5,9,200 reviews testify. These reviews give detailed information about the development of oxaziridine chemistry and the use of some oxaziridines as oxidizing reagents. In contrast to the vigorous development of the chemistry of dioxiranes fewer new reports were published on oxaziridines in recent years. Nevertheless, it is necessary to give some brief information in this monograph about the epoxidation of imines and the utilization of oxaziridines synthesized in this way. The conclusions are based on the information of the above-mentioned reviews.
Extensive investigations of these compounds have revealed their unusual reactivity. This, undoubtedly, is related to the strained three-membered ring and a relatively weak N O bond. A consequence of these features is the low basicity of the oxaziridine nitrogen compared to that of amines. Another remarkable property of some oxaziridines is that they possess a configurationally stable nitrogen atom at ordinary temperatures200.
It is important to underline in this introduction that the importance of oxaziridines as special oxidizing agents is expected to diminish in some fields due to the use of dioxiranes. Their importance, however, is indisputable since oxaziridines as chiral oxidizing agents201,202 offer greater possibilities than dioxiranes.
B. Preparation of Oxaziridines
The most common method for the epoxidation of imines is the peracid203 205 and oxone206 210 oxidation. Two mechanisms have been put forward: the concerted mechanism (analogous to an olefin epoxidation) and the two-step mechanism proceeding through an intermediate. On the basis of recent investigations the latter mechanism seems to be more likely (equation 40)204,208,211.
|
R3 |
|
|
|
|
|
|
|
|
|
R3 |
||
|
N |
|
|
O |
H |
R3 |
|
|
|
N |
|||
|
|
|
N |
|
|
|
|
|
O |
||||
|
|
R4 CO3 H |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
R4 |
|
|
|
|
−R4 COOH R1 |
R2 |
||
R1 |
R2 |
|
|
O |
R2 |
|
|
||||||
|
|
|
|
|
|
|
R1 |
|
|
|
|
||
40
