
23. The thiocarbonyl group |
1405 |
yielding a thioketone236, which is very prone to undergo intramolecular [4C2] cycloadditions even at room temperature (equation 16).
Li |
Cl |
|
Cl2 CS |
C S |
|
−30 °C |
SnMe3 |
|
71% |
||
|
||
|
cat. BF3 .Et2 O |
|
|
−Me3 SnCl |
(16)
C S
46%
The reaction of phosphorus ylides with elemental sulfur gave thioaldehydes, which were trapped in situ by treatment with secondary amines to afford the corresponding thioamides (equation 17)237.
|
|
|
H |
|
Ph3 P |
|
CHR + S8 |
S C |
amine |
|
|
|||
|
|
|||
|
||||
|
|
|
R |
|
NR′2
S C
R
(17)
47−76%
R = CO2 Me, CO2 Et
Amine: morpholine, dimethylamine, piperidine, pyrrolidine.
The transient thioaldehydes can also be trapped with dienes giving the corresponding Diels-Alder adducts in good yields. These reactions are carried out in toluene under reflux. Milder conditions (reflux in dichloromethane or benzene) have been reported by Sato and Satoh for a similar transformation which involves cyclic polysulfides instead of elemental sulfur238 (equation 18). The aldehydes obtained were trapped in Diels-Alder reactions.
R1 |
S |
Ph3 P |
+ |
H |
S |
(S)n |
|
|
n = 2, 3 |
R1
CH2 Cl2 or C6 H6
S (18)
−Ph3 PS
H
1406 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
B. Addition of Sulfur to Carbenes
The ethylene linkage in dithioflavylene can be cleaved either by sulfur under drastic conditions (280 °C) or photolytically (under reflux in toluene) giving higher yields239 (equation 19).
X |
Ph |
X |
Ph |
|
hν |
|
|
|
S |
|
(19) |
|
|
S |
|
|
2 |
|
|
X=O, S |
|
63−68% |
|
C. Thionation of Carbonyl Derivatives
1. Carbonyl compounds
Thionation of carbonyl groups is the most frequently used route for the obtention of thiocarbonyl compounds and many methods are known to achieve this transformation223. Brillon240 has reviewed the different reagents employed in the O/S exchange, which traditionally has been accomplished by using inorganic sulfides, but in recent years Lawesson reagent241,242 has become the method of choice.
a. Hydrogen sulfide (H2 S). The use of hydrogen sulfide in the presence of an acid catalyst, usually hydrogen chloride, is a classical method for the preparation of thiocarbonyl compounds. The reaction proceeds by reversible protonation of the carbonyl group, which facilitates substitution at carbon by H2S to give a mercapto hydroxy hemiacetal which eliminates to afford the thiocarbonyl group (equation 20).
|
O |
|
|
|
OH |
|
|
|
|
S |
|||||
|
|
H2 S, H+ |
|
|
|
|
|
R2 |
|
H+, −H3 O+ |
|
||||
|
|
|
|
|
R1 |
|
C |
|
|
|
|
(20) |
|||
R1 |
R2 |
|
|
||||||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
R1 |
R2 |
|||||
|
SH |
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This exchange process is acid-catalyzed in both steps and special care must be taken to avoid polymerization of the final products. This is usually effected by working at low temperatures, when good yields and clean products are obtained.
This method has been applied to the preparation of enaminothioketones243 although sometimes the yields are very low (30%)244. The dimerization product is obtained in the reaction of fluorenone with H2S/HCl245, and the pyrolysis of the dimer leads to the hydrocarbon rubicene (equation 21). However, almost quantitative yields of monomeric thiofluorenone can be obtained by performing the reaction at lower temperatures and with higher concentrations of H2S/HCl246.
23. The thiocarbonyl group |
1407 |
HCl
H2 S
O S
S
S
87%
(21)
Naltrexone is an opioid antagonist which has been thionated with the system H2S/HCl at 78 °C to give the crude enethiol (equation 22)247 which is further oxidized to the corresponding disulfide.
Cl− |
+ NH |
Cl− |
+ NH |
|
OH |
|
OH (22) |
|
|
H2 S/HCl |
|
|
|
EtOH |
|
|
O |
O |
|
HO |
O |
HO |
SH |
Although this combination of reagents has not been applied to the preparation of normal aldehydes due to the high instability of these compounds, it has proven very useful in the obtention of some heterocyclic thioaldehydes, especially those belonging to the pyrazole and indole series248 (equation 23). The authors claim that yields are higher than using Lawesson reagent and, if present, concomitant reduction of the azido group takes place.
The problem of the synthesis of thioaldehydes has been partly circumvented in recent years by using silyl thioketones as their synthetic equivalents. This clever methodology was introduced by Italian chemists and they have successfully exploited many different applications of these compounds, which have been reviewed by Bonini249. In this

1408 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
R2 |
|
|
CHO |
|
|
R2 |
|
|
CHS |
R2 |
|
|
CHO |
|
|
N |
|
|
H2 S/MeOH |
|
N |
|
|
|
H2 S/MeOH |
|
N |
|
|
|
|
|
|
|
|
|
|
HCl |
|
|
||||
|
|
NH2 |
HCl |
|
|
|
NH2 |
|
N3 |
|||||
|
N |
|
|
N |
N |
|||||||||
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
||||||
|
R |
1 |
|
|
55% |
R |
1 |
|
44−80% |
R |
1 |
|
||
|
|
|
|
|
|
|
|
|
|
|||||
R1, R2 = Me, Ph, t-Bu |
(23) |
|
approach, acylsilanes are converted into silyl thioketones by using H2S/HCl. The thiones, with different stabilities, were trapped in situ with 1,3-dienes affording the corresponding Diels-Alder cycloadducts, which then can be desilylated with tetrabutylammonium fluoride (TBAF) to give the thioaldehyde adduct (equation 24), thus proving the synthetic equivalence of silicon with an achiral or even ‘chiral’ proton250.
|
O |
S |
S |
|
H2 S/HCl |
|
|
R |
SiMe3 |
R |
SiMe3 |
|
|
|
R |
|
|
|
SiMe3 |
|
R = alkyl, aryl |
|
TBA F |
|
|
|
S |
|
|
|
R |
(24)
In an extension of this method, ω-haloacylsilanes were transformed by H2S/HCl into the corresponding silyl thiones, which underwent enethiolization on base treatment and subsequent intramolecular cyclization to afford a range of cyclic sulfides251.
b. Phosphorus decasulfide (P4S10). This compound, also known as phosphorus pentasulfide P2S5, is the phosphorus-based inorganic reagent most frequently employed and its use for the sulfurization of carbonyl groups has spanned over a century. The major problem associated with P4S10 is its poor solubility in organic solvents at 25 °C. Therefore, thionation reactions with P4S10 are generally performed at high temperature in HMPT, pyridine or o-dichlorobenzene. Taking into account the mechanism proposed for this reagent252, several in situ derivatives obtained by combination of P4S10 with sodium bicarbonate or sodium carbonate have been introduced240.
In recent years, P4S10 has been successfully applied to the synthesis of bridged thioketones (adamantanethione, thiofenchone and thiocamphor)253 and other bicyclic thiones254 (equation 25).
The synthesis of organic metals containing either a tetrathiafulvalene (TTF) system255 or a trimethylenemethane (TMM) system256 (equation 26) has been accomplished by thionation with P4S10.
|
23. The thiocarbonyl group |
|
1409 |
||
O |
|
O |
|
|
S |
|
P4 S10 |
|
|
|
+ |
|
xylene |
|
|
|
|
|
|
|
|
|
|
O |
|
|
S |
|
S |
|
|
|
|
|
(25) |
Me |
Me |
|
Me |
|
Me |
S |
S |
P4 |
S10 |
S |
S |
|
|
|
(26) |
||
|
|
benzene |
|
||
|
|
|
|
||
O |
O |
|
S |
|
S |
|
|
58% |
|
|
|
P4S10 has also been utilized in the preparation of 1H-pyridine-4-thiones, useful in the synthesis of modified cephalosporins257. However, attempted thionation of diacylketene thioacetals with P4S10 Et3N in acetonitrile at low temperature afforded the primary dealkylation product and not the thionated one258. Azulenyl thioketones (equation 27a)259 as well as tropothione derivatives260,261 bearing an electron-donating group at the C-2 position (equation 27b) (see also Section II) have also been obtained by thionation with P4S10. In the latter syntheses, the use of ‘polar’ solvents (dichloromethane, acetonitrile) was crucial for the success of the reaction. These substituted tropothiones are thermally stable and do not dimerize in the solid state at room temperature.
O S
P4 S10 (27a)
P4 S10
O  S
S
Et3 N
(27b)
R R
R = H, Me, Ph, NH2 , NHMe, OH, OMe, SMe
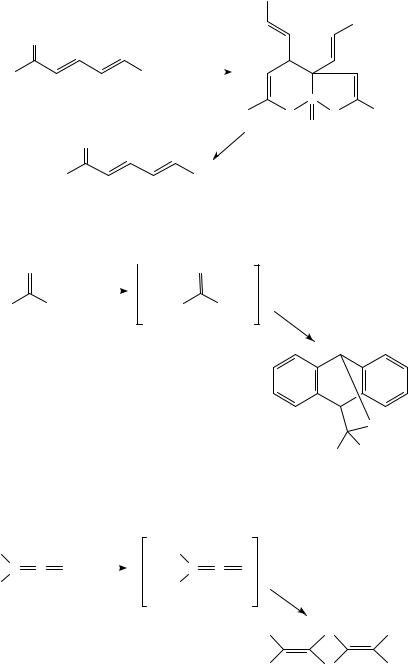
Treatment of dienyl aryl ketones with P4S10 affords phosphorus-containing heterocycles which generate dienyl aryl thioketones upon heating262 (equation 28).
1410 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
Ph |
O |
|
|
|
|
|
|
Ar |
Ph |
P4 S10 |
|
|
|
|
|
|
|
|
|
||
Et3 N, CS2 |
|
|
(28) |
|||
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
P |
|
|
|
|
Ar |
S |
S |
Ar |
|
|
|
|
|
S |
|
S |
|
|
∆ |
|
|
|
|
|
|
|
|
|
|
Ar |
|
Ph |
|
|
|
|
In a related approach, |
˛, ˇ-unsaturated thioketones, |
formed |
in situ |
by thionation |
||
with P4S10 of the corresponding ketones, undergo intramolecular hetero-Diels-Alder reactions263. Diethyl thioxomalonate was formed from diethyl oxomalonate and phosphorus pentasulfide (equation 29) and was trapped as a Diels-Alder adduct264.
|
O |
|
|
|
|
S |
|
P4 S1 0 |
|||||
|
|
|
||||
EtO2 C |
CO2 Et |
|
|
|
EtO2 C |
CO2 Et |
|
|
|
||||
anthracene
|
S |
EtO2 C |
CO2 Et |
|
(29)
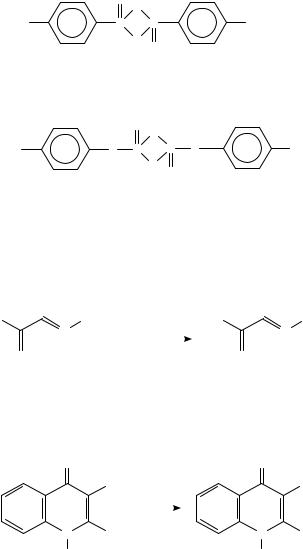
Attempts to prepare a halogenated thioketene by refluxing a number of different precursors with P4S10 in toluene yielded only the cyclic dimer and the corresponding 1,3,4-trithiolan (equation 30)265.
CF3 S |
|
CF3 S |
|
|
|
P4 S10 |
|
|
|
C C O |
|
C C S |
|
|
|
|
|
||
CF3 S |
toluene |
|
|
|
|
CF3 S |
|
|
|
|
|
CF3 S |
S |
SCF3 |
|
|
CF3 S |
S |
SCF3 |
|
|
|
|
(30) |
23. The thiocarbonyl group |
1411 |
c. Phosphetane compounds: Lawesson reagent. Lawesson reagent 52241,242, very efficient, versatile and soluble in benzene, toluene and xylene, has replaced most of the classical reagents discussed above. Other useful media are THF, HMPT, DME or o- dichlorobenzene. The mechanism of thionation with Lawesson reagent has been studied266 and different analogs such as Belleau’s reagent267 (53) or Yokoyama’s reagents268 (54, 55) with increased solubility in these solvents have been prepared.
S
S
RO |
P |
P |
OR |
S
S
(52)R = CH3
(53)R = Ph
S
S
R S P P S R
S
S
(54)R = H
(55)R = OCH3
Lawesson reagent is the most widely used for thionation reactions and has been applied to a wide variety of syntheses240 such as those of camphorthione269, enaminothiones270, thiochromone271 and different diaryl and aryl alkyl thiones272. In addition, hydrazonothioacetophenones have been prepared from the corresponding carbonyl compounds under controlled conditions273. The heterodienes thus obtained underwent [4 C 2] cycloadditions (see Section IV.E.4) affording different heterocyclic systems (equation 31).
Ar |
NR2 |
Ar |
|
NR2 |
|
N |
|
52, benzene |
|
|
N |
O |
|
10° C → rt |
|
S |
(31) |
|
|
|
|||
|
|
|
|
||
NR2 = NMe2 , piperidine.
Ar = Ph, 4-BrC6 H4 , 4-ClC6 H4
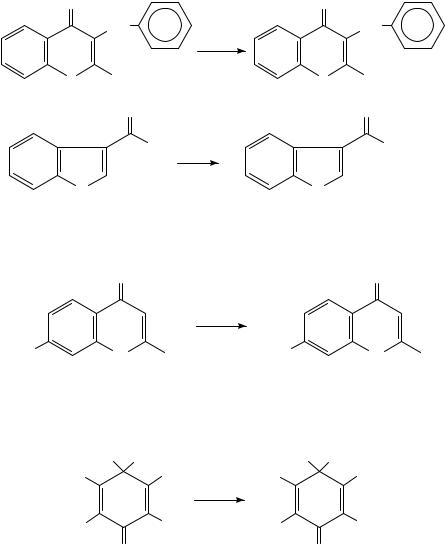
Quinoline-4-thiones274 (equation 32), 3-benzyl-1,4-dithiochromones275 (equation 33),
4-thioisoflavones276 and different pyrrole277 and indole derivatives277 |
|
279 (equation 34) |
|||
|
|||||
have also been obtained with Lawesson reagent. |
|
|
|
||
O |
|
|
S |
|
|
|
R1 |
|
|
R1 |
|
|
52 |
|
(32) |
||
|
|
pyridine |
|
||
|
|
|
|
|
|
N |
R2 |
N |
|
R2 |
|
Me |
|
|
Me |
|
|
1412 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
O |
|
S |
|
|
|
CH2 |
|
CH2 |
|
|
|
52 |
|
|
|
|
toluene |
|
|
S |
R |
S |
R |
(33) |
|
|
|
|
|
|
O |
|
S |
|
|
Me |
52 |
Me |
(34) |
|
|
|
||
|
|
|
|
|
|
|
DME |
|
|
|
N |
N |
|
|
|
H |
H |
|
|
Even tellurium analogs of chromones and flavones have been prepared with Lawesson reagent280 (equation 35).
|
O |
|
|
S |
|
|
|
|
52 |
|
(35) |
|
|
|
|
|
|
|
|
|
toluene |
|
|
MeO |
Te |
Ph |
MeO |
Te |
Ph |
Reagent 52 has also been very successful in the preparation of highly conjugated systems such as cyclohexadienethiones281 (equation 36) and cyclobutanethiones282 (equation 37). In the latter example, depending on the reaction conditions, it is possible to isolate the monoor the dithione.
Ph |
CN |
Ph |
CN |
Ph |
Ph |
Ph |
Ph |
|
52 |
|
|
|
toluene |
|
(36) |
R |
Br |
R |
Br |
|
O |
|
S |
R = H, Br
The thiocarbonyl group is a classic bioisosteric replacement for the carbonyl group which has been widely exploited in medicinal chemistry. This is illustrated with the preparation of thioketones derived from thiocolchicine283 and isothiocolchicine284 which exhibited high antitubulin activity (equation 38).
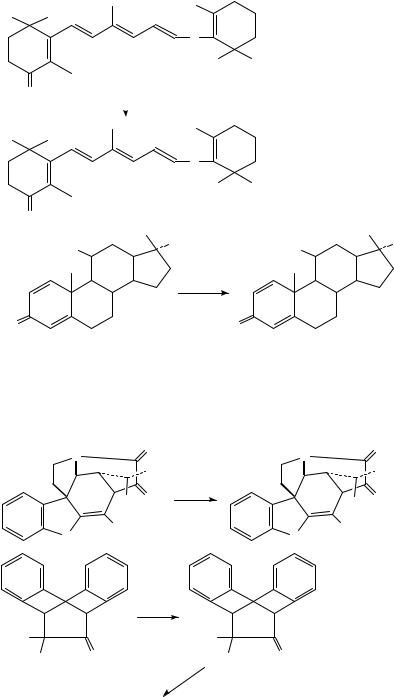
Again in the field of natural products synthesis, Lawesson reagent has found application in the obtention of sulfur carotenoids285 (equation 39) and thionated steroids286 (equation 40).

23. The thiocarbonyl group
O S
52
toluene
O O
+
S
S
MeO
NHCOCH3
MeO
MeO
O
SMe
toluene, r.t 52
MeO
NHCOCH3
MeO
MeO
S
1413
(37)
(38)
39% SMe
1414 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
R
O |
benzene |
52 |
|
40°C |
|
|
|
(39) |
R
S
|
R1 |
|
R1 |
R3 |
R2 |
R3 |
R2 |
|
|
(40)
52, THF
r.t. 32−96%
O S
The versatility and selectivity of the Lawesson reagent is also evidenced in the synthesis of the highly complex alkaloid 19-hydroxytubotaiwine287, in which a ketolactam is transformed into a thionethiolactam (equation 41). Also, Diels-Alder cycloadducts can be thionated with 52 and, upon thermolysis at high temperatures (FVT), afford the corresponding thioketenes which are trapped in situ288 (equation 42).
|
O |
|
|
S |
|
|
N |
|
|
N |
|
|
OH |
|
|
OH |
|
|
O |
52 |
|
S |
(41) |
|
toluene |
|
|||
|
|
|
|
||
|
CO2 Me |
|
|
CO2 Me |
|
|
N |
|
|
N |
|
|
H |
|
|
H |
|
|
52 |
|
|
|
|
Me |
toluene |
Me |
|
(42) |
|
|
|
|
|
||
Me |
O |
|
Me |
S |
|
|
|
FVT |
|
|
|
|
|
700 °C |
|
|
|
[Me2 C C
C S]
S]
